Molecular Photonics
Key Publications
2019 Publications
- Swick, S.; Gebraad, T.; Jones, L.; Fu, B.; Aldrich, TJ.; Kohlsted, KL.; Schatz, GC.; Facchetti, A.; Marks, TJ.; Building Blocks for High-Efficiency Organic Photovoltaics. Molecular, Crystal, and Electronic Characteristics of Post-Fullerene ITIC Ensembles, ChemPhysChem, 2019, in press.
- Chen, Y.; Zhu, W.; Wu, J.; Huang, Y.; Facchetti, A.; Marks, T.J.; Recent Advances in Squaraine Dyes for Bulk-Heterojunction Organic Solar Cells, Org. Photonics Photovolt.2019, 7, 1-16. DOI: 10.1515/oph-2019-0001.
- Manley, E.F.; Harschneck, T.; Eastham, N.D.; Leonardi, M.J.; Zhou, N.; Chang, R.P.H.; Chen, L.X.; Marks, T.J.; Side Chain and Solvent Direction of Film Morphology in Small-Molecule Organic Solar Materials. In situ Analysis, Chem. Mater. 2019, in press.
- Lou, A. J.-T.; Marks, T.J.; A Twist on Nonlinear Optics: Understanding the Unique Response of π-Twisted Chromophores, Accts. Chem. Res., 2019, 52, 1428-1438. DOI: 10.1021/acs.accounts.9b00077.
- Aldrich, T.J.; Matta, M.; Zhu, W.; Swick, S.M.; Stern, C.; Schatz, G.C.; Facchetti, A.; Melkonyan, F.S.; Marks, T.J.; Fluorination Effects on Indaceno-dithienothiophene Acceptor Packing and Electronic Structure, End-Group Redistribution, and Solar Cell Photovoltaic Response, J. Am. Chem. Soc. 2019, 141, 3274–3287. DOI: 10.1021/jacs.8b13653.
- Aldrich, T.J.; Zhu, W.; Mukherjee, S.; Richter, L.J.; Gann, E.; DeLongchamp, D.M.; Facchetti, A.; Melkonyan, F.S.; Marks, T.J.; Stable Postfullerene Solar Cells via Direct C–H Arylation Polymerization. Morphology–Performance Relationships, Chem. Mater. 2019, 31, 4313-4321. DOI: 10.1021/acs.chemmater.9b01741
- Wang, G.; Melkonyan, F.; Facchetti, A.; Marks, T.J.; All-Polymer Solar Cells: Recent Progress, Challenges, and Perspectives, Angew. Chem. Int. Ed. 2019, 58, 4129-4142. DOI: org/10.1002/ange.201808976.
- Wang, G.; Swick, S.; Matta, M.; Mukherjee, S.; Chen, Y.; Logsdon, J.L.; Fabiano, S.; Huang, W.; Aldrich, T.J.; Yang, T.; Timalsina, A.; Powers-Riggs, N.; Young, R.M.; Wasielewski, M.R.; Kohlstedt, K.L.; DeLongchamp, D.M.; Schatz, G.C.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J.; Directing Photovoltaic Blend Microstructure for Higher Efficiency Post-Fullerene Solar Cells. To Tilt or not to Tilt?J.Am. Chem. Soc. 2019,141, 34, 13410-13420. DOI: 10.1021/jacs.9b03770.
Previous Publications
- imalsina, A.; Hartnett, P. E.; Melkonyan, F. S.; Strzalka, J.; Reddy, V. S.; Facchetti, A.; Wasielewski, M. R.; Marks, T. J., New donor polymer with tetrafluorinated blocks for enhanced performance in perylenediimide-based solar cells. J. Mater. Chem. A 2017, 5(11), 5351-5361.
- Zhou, N.; Dudnik, A. S.; Li, T. I. N. G.; Manley, E. F.; Aldrich, T. J.; Guo, P.; Liao, H.-C.; Chen, Z.; Chen, L. X.; Chang, R. P. H.; Facchetti, A.; Olvera de la Cruz, M.; Marks, T. J., All-Polymer Solar Cell Performance Optimized via Systematic Molecular Weight Tuning of Both Donor and Acceptor Polymers. J. Am. Chem. Soc. 2016, 138 (4), 1240-1251.
- Melkonyan, F. S.; Zhao, W.; Drees, M.; Eastham, N. D.; Leonardi, M. J.; Butler, M. R.; Chen, Z. H.; Yu, X. G.; Chang, R. P. H.; Ratner, M. A.; Facchetti, A. F.; Marks, T. J., Bithiophenesulfonamide Building Block for pi-Conjugated Donor-Acceptor Semiconductors. J. Am. Chem. Soc. 2016, 138 (22), 6944-6947.
- Dudnik, A. S.; Aldrich, T. J.; Eastham, N. D.; Chang, R. P. H.; Facchetti, A.; Marks, T. J., Tin-Free Direct C–H Arylation Polymerization for High Photovoltaic Efficiency Conjugated Copolymers. J. Am. Chem. Soc. 2016, 138 (48), 15699-15709.
- Hartnett, P. E.; Timalsina, A.; Matte, H. S. S. R.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R. P. H.; Hersam, M. C.; Wasielewski, M. R.; Marks, T. J., Slip-Stacked Perylenediimides as an Alternative Strategy for High Efficiency Nonfullerene Acceptors in Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136 (46), 16345-16356.
- Guo, X. G.; Zhou, N. J.; Lou, S. J.; Smith, J.; Tice, D. B.; Hennek, J. W.; Ortiz, R. P.; Navarrete, J. T. L.; Li, S. Y.; Strzalka, J.; Chen, L. X.; Chang, R. P. H.; Facchetti, A.; Marks, T. J., Polymer solar cells with enhanced fill factors. Nat Photonics 2013, 7 (10), 825-833.
Novel Electron Transporting Materials
The development and incorporation of novel electron-transporting materials are important in studying and improving the efficiency of organic photovoltaics. The Marks group, in collaboration with other groups, has successfully incorporated new polymer materials as the donor with a series of non-fullerene acceptors as the electron transporting material, with power conversion efficiency over 10%. And from the fundamental structure of the active layer film, the Marks group is trying to understand the structure-property relationship of active layer material. The B Team also collaborates with the D Team in studying the new materials, both n- and p-type, that D Team members develop for organic field-effect transistors that show promising characteristics for organic photovoltaics. In addition, B Team members are involved in the design and synthesis of new small molecule acceptors (IT derivatives, Scheme 1) for organic photovoltaics to replace the current industry standard fullerene.
Scheme 1. Synthetic Route to IT derivatives: ITN-C12 and ITzN-C9
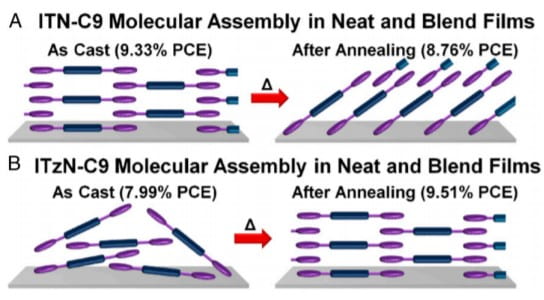
Figure 1. Schematic representation of the morphological changes observed with annealing neat and PBDB-TF blend films of (A) ITN-C9 and (B) ITzN-C9
Fluorination Effects on Non-Fullerene Acceptor
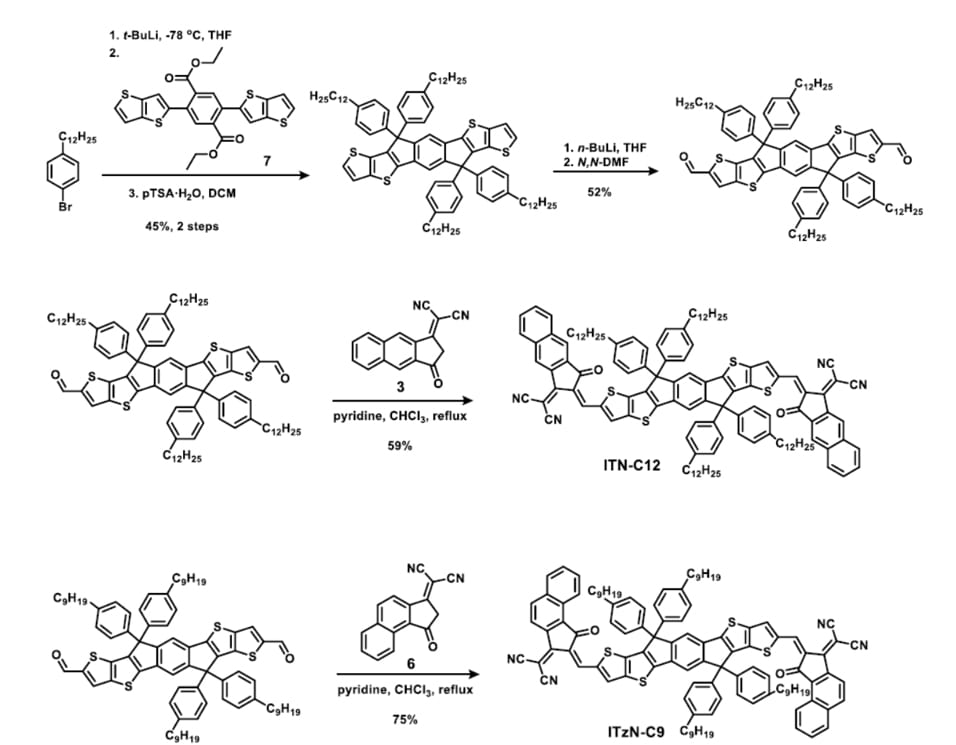
End-group fluorination has been an effective strategy to enhance the power conversion efficiency of OPV devices fabricated by bulk heterojunction design. Specifically, Indacenodithienothiophene (IDTT)-based postfullerene electron acceptors, such as ITIC are one of the outstanding examples that benefit from this strategy. The Marks group has systematically investigated the influence of end-group fluorination density, and positioning on the physicochemical properties, single-crystal packing of the IDTT based acceptors. Also, combining with the single-crystal packing features, we found that the present ITIC-nF acceptors containing thetrifluorinated end-group (ITIC-3F and ITIC-6F) achieve superior PV performance metrics (over 13%) versus the analogous acceptors containing the difluorinated end-group (ITIC-2F and ITIC-4F).
Figure 2. Single-crystal structures of (a) ITIC-0F, (b) ITIC-4F, and (c) ITIC-6F
Figure 3. Comparison of the PBDB-TF:ITIC-nF binary and ternary PSC photovoltaic performances. (a) J−V response of the binary PSCs and (d)
corresponding EQE spectra. (b) Comparison of the J−V response for the scrambled (SCR) and unscrambled binary PSCs and (e) corresponding
EQE spectra. (c) Comparison of the J−V response for the scrambled (SCR) and unscrambled ternary PSCs and (f) corresponding EQE spectra.
Electron Blocking Layers in BHJ Solar Cells
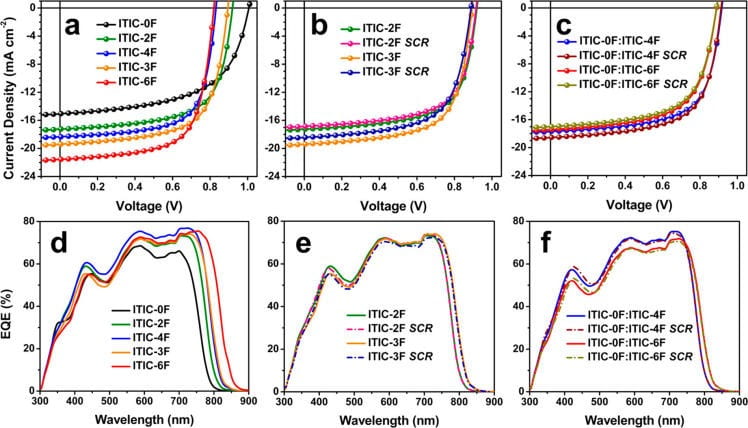
The electron donor (hole transporter) and electron acceptor (electron transporter) are intermixed in bulk heterojuction cells. Such intermixing is extremely important for optimal charge separation but it unfortunately provides opportunities for the charges to go to the wrong electrodes because both the electron and the hole transporters are in physical contact with both the electrodes. In order to prevent the electrons from going to anode, we add an interfacial layer between the anode and the active material. Because of the function it serves in the cells, we call it the electron blocking layer (EBL).
The Mark’s Group has designed some really interesting EBL materials in the past couple of years. TPDSi 2 is a molecule that was first developed in the Marks Group as an EBL in OLEDS but it also worked really well as an EBL in MDMO-PPV cells. The energy level of a TPDSi 2-TFB composite layer makes is a good EBL for such cells.
Another EBL that we have designed is the inorganic Nickel Oxide (NiO) layer. The energetics of NiO and donor polymer P3HT match well.The EBL we have designed affords higher efficiencies and better lifetimes over the industry standard PEDOT:PSS as EBL.
Organic photovoltaic (OPV) modeling
We develop device models, defining and quantifying loss mechanisms in OPVs. If an OPV cell performs at 5% efficiency, what exactly is happening to the other 95%? We are currently studying key loss mechanisms (e.g., exciton dissociation, charge recombination), developing more specific models of these processes, and determining how changes to particular materials parameters (e.g., conductivity, dielectric constant, etc.) can enhance these processes and, ultimately, overall OPV power conversion efficiency.

Figure 4. Evaluation of the effects anode conductivity and cell size have on power conversion efficiency. Note that the zero loss case (upper left hand corner of graph) has a power conversion efficiency of 13.6%