Developing COVID-19 Vaccines (CDC)
Bringing a new vaccine to the public involves many steps including vaccine development, clinical trials, US Food and Drug Administration (FDA) authorization or approval, manufacturing, and distribution. Many different public organizations and private companies have worked together to make COVID-19 vaccines available to the public. While COVID-19 vaccines have been developed rapidly, all steps have been taken to ensure their safety and effectiveness.
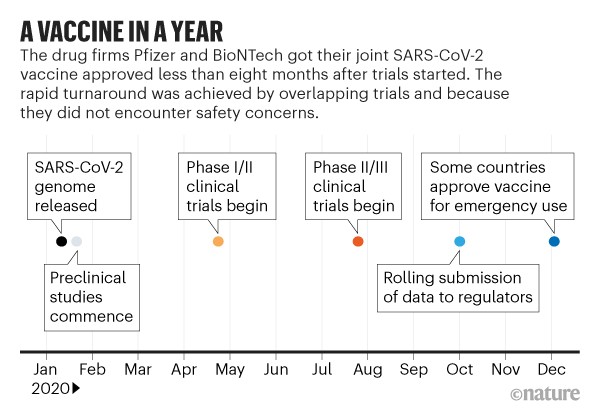
The lightning-fast quest for COVID vaccines (Nature)
The world was able to develop COVID-19 vaccines so quickly because of years of previous research on related viruses and faster ways to manufacture vaccines, enormous funding that allowed firms to run multiple trials in parallel, and regulators moving more quickly than normal. Some of those factors might translate to other vaccine efforts, particularly speedier manufacturing platforms.
Here’s How It Was Possible to Develop COVID-19 Vaccines So Quickly (healthline)
Resources, public support, existing technology, and more all contributed to the speed of vaccine development.
How COVID-19 Vaccines Get to You (CDC)
Vaccine manufacturers; the federal government; state, local, and territorial jurisdictions; and other partners are working to make sure safe and effective vaccines are getting to you as quickly as possible. This page will help you understand the key steps in this important process and how CDC is tracking vaccine distribution, delivery, and administration throughout the United States.

