Publications
| 94. | Heavy Atom Quantum Mechanical Tunneling in Total Synthesis, Guo, W.; Robinson, E. R.; Thomson, R. J.; Tantillo, D. J.Org. Lett. 2024, 26, 4606–4609. |

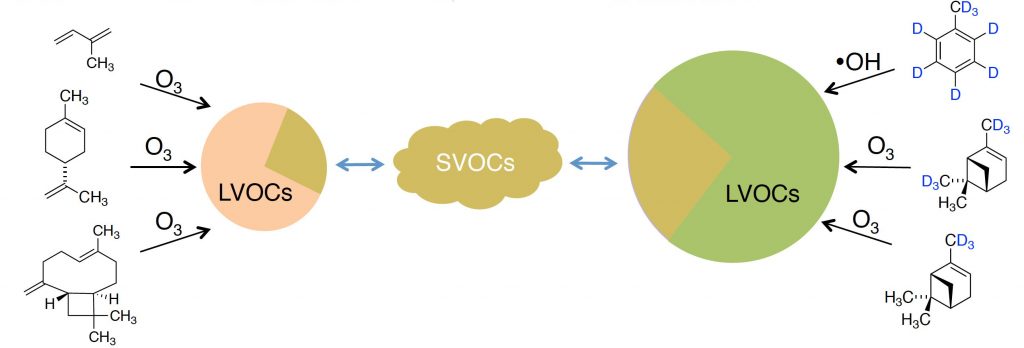
| 93. | Selective Deuteration as a Tool for Resolving Autoxidation Mechanisms in α-Pinene Ozonolysis, Meder, M.; Peräkylä, O.; Varelas, J. G.; Luo, J.; Cai, R.; Zhang, Y.; Kurtén T.; Riva, M.; Rissanen, M.; Geiger, F. M.; Thomson, R. J.; Enh, M. Atmos. Chem. Phys. 2023, 23, 4373–4390. |

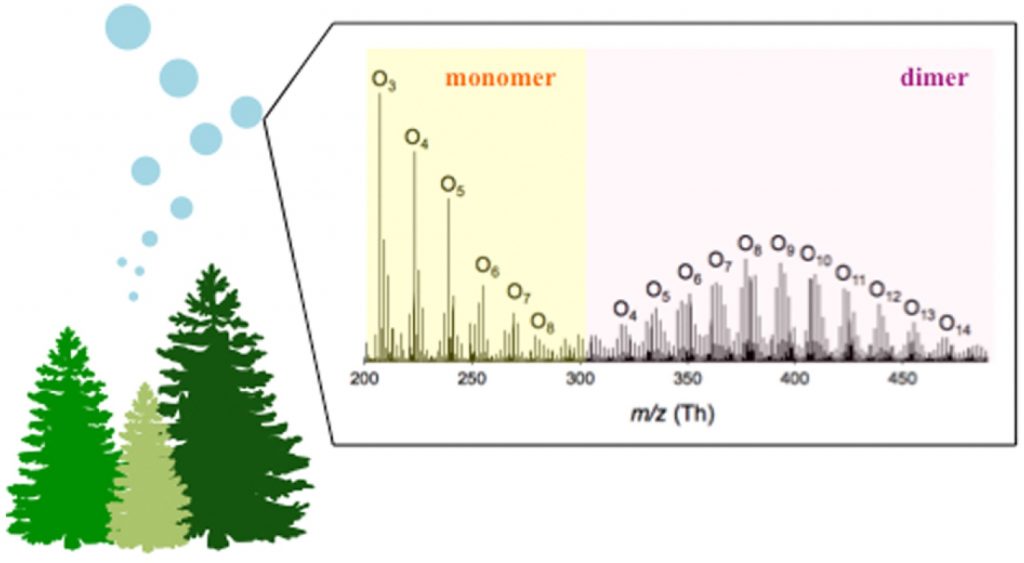
| 92. | A Large Gas-phase Source of Esters and other Accretion Products in the Atmosphere, Peräkylä, O.; Berndt, T.; Franzon, L.; Hasan, G.; Meder, M.; Valiev, R. R.; Daub, C. D.; Varelas, J. G.; Geiger, FS. M.; Thomson, R. J.; Rissanen, M.; Ehn, M. J. Am. Chem. Soc. 2023, 145, 7780–7790. |

| 91. | Organic Synthesis in the Study of Terpene-derived Oxidation Products in the Atmosphere, Upshur, M. A.; Bé, A.. G.; Luo, J.; Varela, J. GD.; Geiger, F. M.; Thomson, R. J. Nat. Prod. Rep. 2023, 40, 890–921. |

| 90. | Ring-opening Yields and Auto-oxidation Rates of the Resulting Peroxy Radicals from OH-oxidation of α-Pinene and β-Pinene, Lee. B. H.; Iyer, S.; Kurtén, Varelas, J. G.; Luo, J.; Thomson, R. J.; Thornton, J. A. Environ. Sci.: Atmos.. 2023, 3, 399–407. |

| 89. | Synthesis Enabled Investigations into the Acidity and Stability of Atmospherically-Relevant Isoprene-Derived Organosulfates, Varelas, J. G.; Vega, M. M.; Upshur, M. A.; Geiger, F. M.*; Thomson, R. J.* ACS Earth Space Chem. 2022, 6, 3090–3100. |

| 88. | Unanticipated Hydrophobicity Increases of Squalene and Human Skin Oil Films Upon Ozone Exposure, Butman, J. L.; Thomson, R. J.; Geiger, F. M. J. Phys. Chem. B 2022, 126, 9417–9423. |

| 87. | Gas-Particle Uptake and Hygroscopic Growth by Organosulfate Particles, Ohno, P. E.; Wang, J.; Mahrt, F.; Varelas, J. G.; Aruffo, E.; Ye, J.; Qin, Y.; Kiland, K. J.; Bertram, A. K.; Thomson, R. J.*; Martin, S. T.* ACS Earth Space Chem. 2022, 6, 2481–2490. |

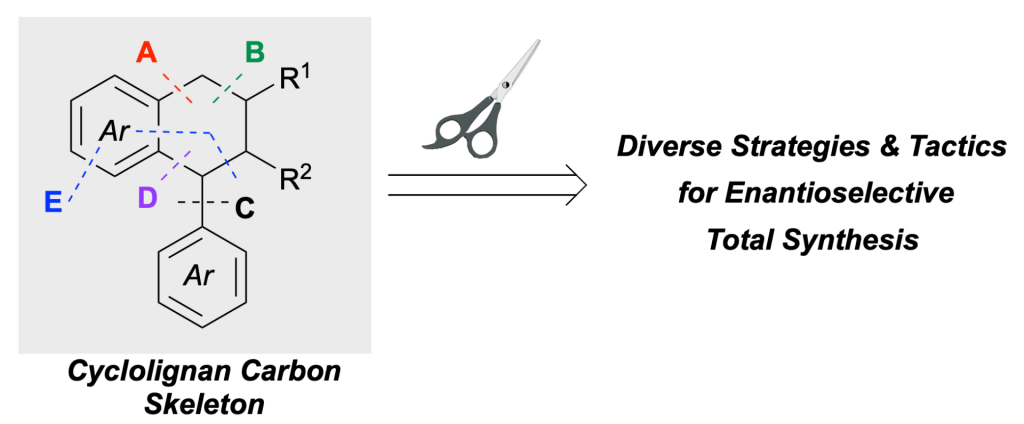
| 86. | Recent Strategies and Tactics for the Enantioselective Total Syntheses of Cyclolignan Natural Products, Reynolds, R, G.; Nguyen, H. Q. A.; Reddel, J. C. T.; Thomson, R. J. Nat. Prod. Rep. 2022, 39, 670–702. |
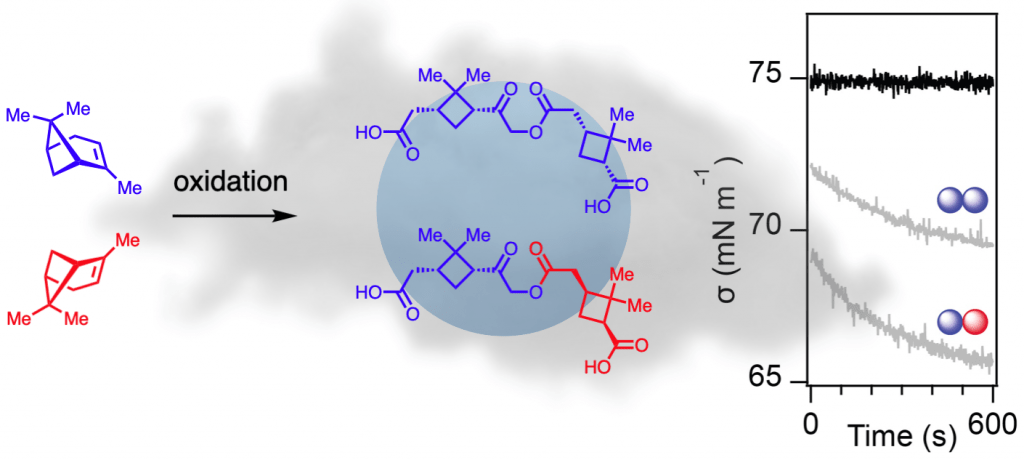
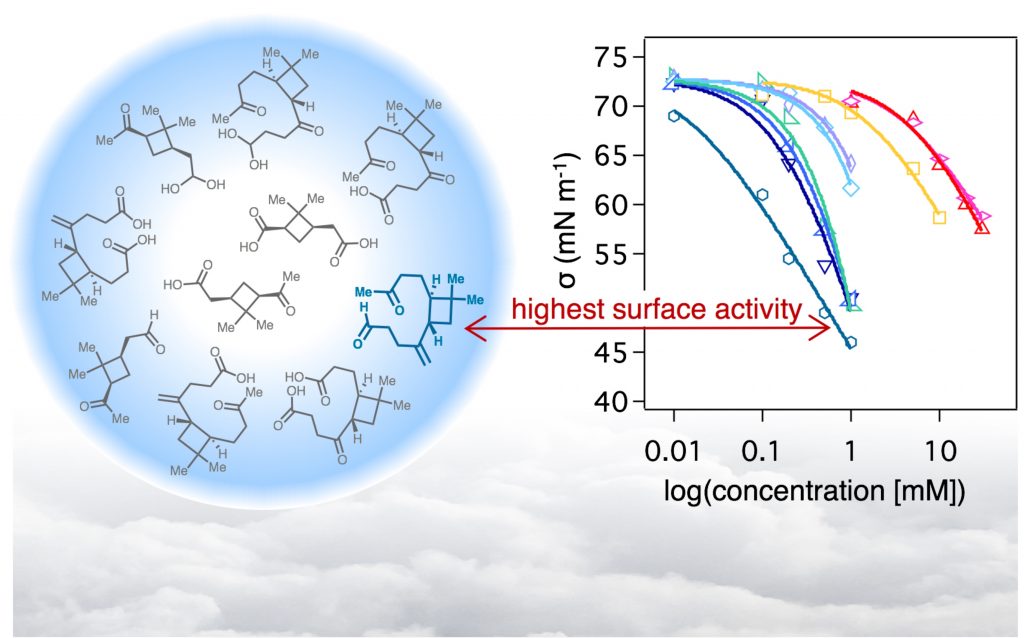
| 85. | Molecular Chirality and Cloud Activation Potentials of Dimeric α-Pinene Oxidation Products, Bellcross, A.; Bé, A. G. ; Geiger, F. M.; Thomson, R. J. J. Am. Chem. Soc. 2021, 143, 16653–16662. |
| 84. | Electrochemical and Photocatalytic Oxidative Coupling of Ketones via Silyl Bis-enol Ethers, Caravana, A. C.; Nagasing, B.; Dhanju, S.; Reynolds, R. G.; Weiss, E. A.; Thomson, R. J. J. Org. Chem. 2021, 86, 6600–6611. |

| 83. | A Community Resource for Paired Genomic and Metabolic Data Mining, Schorn, M. A. et. al. Nat. Chem. Bio. 2021, 17, 363–368. |

| 82. | Streptomyces buecherae sp. nov., an Actinomycete Isolated from Multiple Bat Species, Hamm, P. S.; Dunlap, C. A.; Mullowney, M. W.; Caimi, N. A.; Kelleher, N. L.; Thomson, R. J.; Porras-Alfaro, A.; Northup, D. E. Antonie van Leeuwenhoek 2020, 113, 2213–2221. |

| 81. | Genome Mining and Metabolomics Uncover a Rare D‐Capreomycidine Containing Natural Product and Its Biosynthetic Gene Cluster, Tryon, J. H.; Rote, J. C.; Chen, Li.; Robey, M. T.; Vega, M. M.; Phua, W. C.; Metcalf, W. W.; Ju, K.-S.;Kelleher, N. L.; Thomson, R. J. ACS Chem. Bio. 2020, 15, 3013–3020. |

| 80. | Access to α-Pyrazole and α-Triazole Derivatives of Ketones from Oxidative Heteroarylation of Silyl Enolethers, Dhanju, S.; Caravana, A. C.; Thomson, R. J. Org. Lett. 2020, 22, 8055–8058. |

| 79. | Liquid–Liquid Phase Separation in Organic Particles Consisting of α- Pinene and β-Caryophyllene Ozonolysis Products and Mixtures with Commercially-Available Organic Compounds, Song, Y-C.; Bé, A. G.; Martin, S. T.; Geiger, F. M. Bertram, A. K.; Thomson, R. J.; Song, M. Atmos. Chem. Phys. 2020, 20, 11263–11273. |

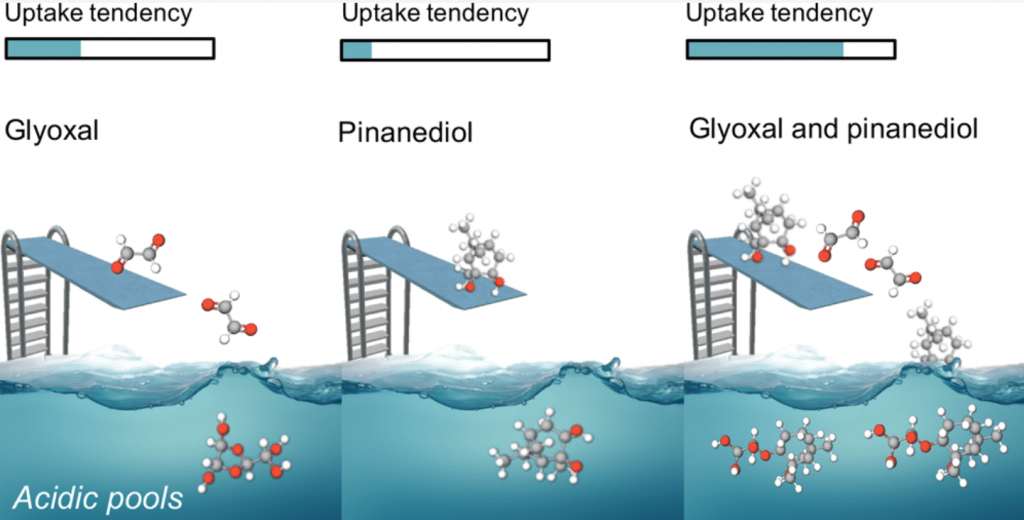
| 78. | Synergistic Uptake by Acidic Sulfate Particles of Gaseous Mixtures of Glyoxal and Pinanediol, Qin, Y.; Ye, J.; Ohno, P. E.; Lei, Y.; Wang, J.; Liu, P.; Thomson, R. J.; Martin, S. T. Environ. Sci. Technol. 2020, 54, 11762–11770. |
| 77. | Ion Mobility Mass Spectrometry as an Efficient Tool for Identification of Streptorubin B in Streptomyces Coelicolor M145, Marshall, A. P.; Johnson, A. R.; Vega, M. M.; Thomson, R. J.; Carlson, E. E. J. Nat. Prod. 2020, 83, 159–163. |
| 76. | A Computational Framework to Explore Large-scale Biosynthetic Diversity, Navarro-Muñoz, J. C.; Selem-Mojica, N.; Mullowney, M. W.; Kautsar, S. A.; Tryon, J. H.; Parkinson, E. I.; De Los Santos, E. L. C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; Roeters, A.; Lokhorst, W.; Fernadez-Guerra, A.; Teresa Dias Cappelini, T.; Goering, A. W.; Thomson, R. J.; Metcalf, W. W.; Kelleher, N. L.; Barona-Gomez, F.; Madema, M. H. Nat. Chem. Bio. 2020, 16, 60–68. |

| 75. | Diene Synthesis by the Reductive Transposition of 1,2-Allenols, Rinaolo, V. J.; Robinson, E. R.; Diagne, A. B.; Schaus, S. E.; Thomson, R. J. Synlett 2019, 30, 2073–2076. |

| 74. | Surface-Active β-Caryophyllene Oxidation Products at the Air/Aqueous Interface, Bé, A. G.; Liu, Y.; Tuladhar, A.; Bellcross, A. D.; Wang, Z.; Thomson, R. J.; Geiger, F. M. ACS Earth Space Chem. 2019, 3, 1740–1748. |
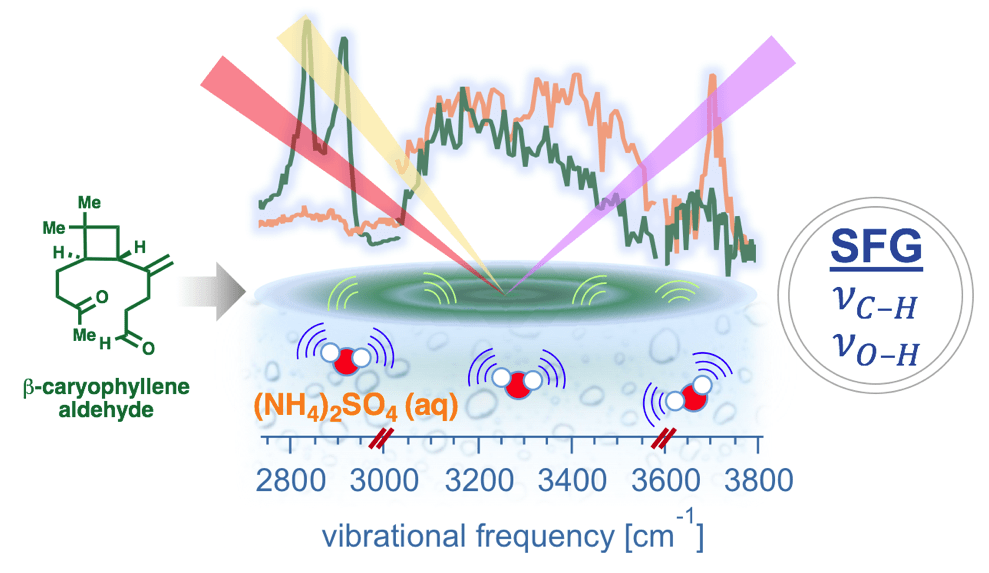
| 73. | Synthesis and Surface Spectroscopy of α-Pinene Isotopologues and their Corresponding Secondary Organic Material, Upshur, M. A.; Vega, M. M.; Bé, A. G.; H. M. Chase; Zhang, Y.; Tuladhar, A.; Chase, Z. A.; Fu, L.; Ebben, C. J.; Wang, Z.; Martin, S. T.; Geiger, F. M.; Thomson, R. J. Chem. Sci. 2019, 10, 8390–8398. |

| 72. | Atmospheric β-Caryophyllene-Derived Ozonolysis Products at Interfaces, Bé, A. G.; H. M. Chase; Liu, Y.; Upshur, M. A.; Zhang, Y.; Tuladhar, A.; Chase, Z. A.; Bellcross, A. D.; Wang, H.-F.; Wang, Z.; Batista, V. S.; Martin, S. T.; Thomson, R. J.; Geiger, F. M. ACS Earth Space Chem. 2019, 3, 158–169. |

| 71. | Canvass: A Crowd-Sourced, Natural-Product Screening Library for Exploring Biological Space, Kearney, S. E. et. al. ACS Cent. Sci. 2018, 4, 1727–1741. |

| 70. | Observations of Sesquiterpenes and their Oxidation Products in Central Amazonia During the Wet and Dry Seasons, Yee, L.D.; Isaacman-VanWertz, G.; Wernis, R.A.; Meng, M.; Rivera, V.; Kreisberg, N. M.; Hering, S. V.; Bering, M. S.; Glasius, M.; Upshur, M. A.; Bé, A. G.; Thomson, R. J.; Geiger, F. M.; Offenberg, J. H.; Lewandowski, M.; Kourtchev, I.; Kalberer, M.; de Sá, S.; Martin, S. T.; Alexander, M. L.; Palm, B. B.; Hu, W.; Campuzano-Jost, P.; Day, D. A.; Jimenez, J. L.; Liu, Y.; McKinney, K. A.; Artaxo, P.; Viegas, J.; Manzi, A.; Oliveira, M. B.; de Souza, R.; Machado, L. A. T.; Longo, K.; Goldstein, A. H. Atmos. Chem. Phys. 2018, 18, 10433–10457. |

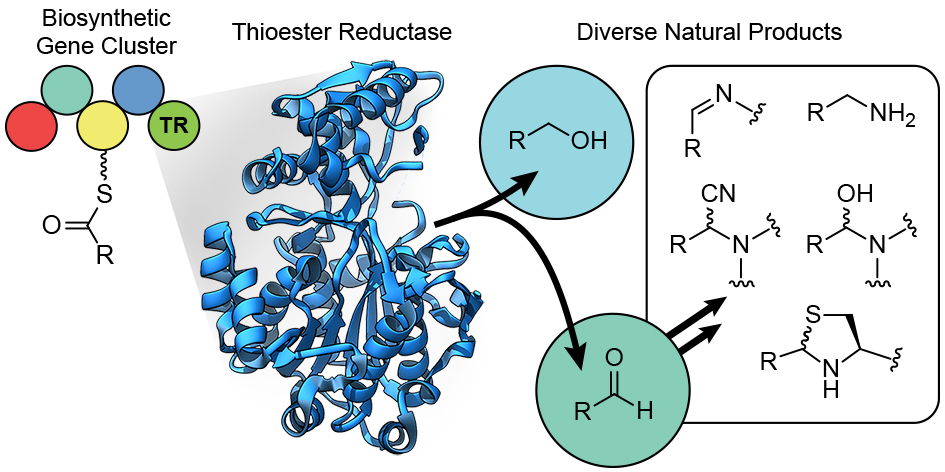
| 69. | Natural Products from Thioester Reductase Containing Biosynthetic Pathways, Mullowney, M. W.; McClure, R. A.; Robey, M. T.; Kelleher, N. L.; Thomson, R. J. Nat. Prod. Rep. 2018, 35, 847–878. |
| 68. | Total Synthesis of Tambromycin Enabled by Indole C–H Functionalization, Miley, G. P.; Rote, J. C.; Silverman, R. B.; Kelleher, N. L.; Thomson, R. J. Org. Lett. 2018, 20, 2369–2373. |

| 67. | Discovery of the Tyrobetaine Natural Products and Their Biosynthetic Gene Cluster via Metabologenomics, Parkinson, E. I.; Tryon, J. H.; Goering, A. W.; Ju, K-S.; McClure, R. A.; Kemball, J. D.; Zhukovsky, S.; Labeda, D. P.; Thomson, R. J.; Kelleher, N. L.; Metcalf, W. W. ACS Chem. Bio. 2018, 13, 1029–1037. |

| 66. | A Strategy for the Convergent and Stereoselective Assembly of Polycyclic Molecules, Robinson, E. R.; Thomson, R. J. J. Am. Chem. Soc. 2018, 140, 1956–1965. |

| 65. | Following Particle–Particle Mixing within Atmospheric Secondary Organic Aerosols Using Isotopically Labeled Terpenes, Ye, Q.; Upshur, M. A.; Robinson, E. S.; Geiger, F. M.; Sullivan, R. C.; Thomson, R. J.; Donahue, N. M. Chem. 2018, 4, 318–333. |
| 64. | Asymmetric Traceless Petasis Borono-Mannich Reactions of Enals: Reductive Transpositions of Allylic Diazenes, Jiang, Y.; Thomson, R. J., Schaus, S. E. Angew. Chem. Int. Ed. 2017, 56, 16631–16635. |

| 63. | Cloud Activation Potentials for Atmospheric α-Pinene and β-Caryophyllene Ozonolysis Products, Bé, A. G.; Upshur, M. A.; Liu, P.; Martin, S. T.; Geiger, F. M.; Thomson, R. J. ACS Cent. Sci. 2017, 3, 715–725. |
| 62. | The Effect of Hydroxyl Functional Groups and Molar Mass on the Viscosity of Non-Crystalline Organic and Organic-Water Particles, Grayson, J. W.; Song, M.; Evoy, E.; Upshur, M. A.; Ebrahimi, M.; Geiger, F. M.; Thomson, R. J.; Bertram, A. K. Atmos. Chem. Phys. 2017, 17, 8509–8524. |

| 61. | Highly Oxygenated Multifunctional Compounds in α‑Pinene Secondary Organic Aerosol, Zhang, X.; Lambe, A. T.; Upshur, M. A.; Brooks, W. A.; Bé, A. G.; Thomson, R. J.; Geiger, F. M.; Surratt, J. D.; Zhang, Z.; Gold, A.; Graf, S.; Cubison, M. J.; Groessl, M.; Jayne, J. T.; Worsnop, D. R.; Canagaratna, M. R. Env. Sci. Technol. 2017, 51, 5932–5940. |
| 60. | Unanticipated Stickiness of α-Pinene, Chase, H. M.; Ho, J.; Upshur, M. A.; Thomson, R. J.; Batista, V. S.; Geiger, F. M. J. Phys. Chem. A. 2017, 121, 3239–3246. |

| 59. | Enantioselective Synthesis of Allenes by Catalytic Traceless Petasis Reactions, Jiang, Y.; Diagne, A. B.; Thomson, R. J.; Schaus, S. E. J. Am. Chem. Soc. 2017, 139, 1998–2005. |
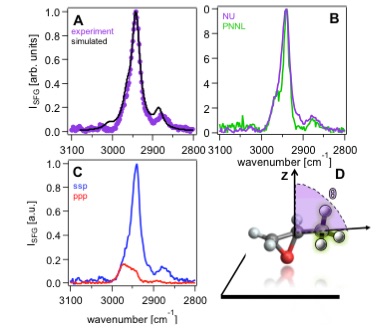
| 58. | Orientations of Nonlocal Vibrational Modes from Combined Experimental and Theoretical Sum Frequency Spectroscopy, Chase, H. M.; Chen, S.; Fu, Li.; Upshur, M. A.; Rudshteyn, B.; Thomson, R. J.; Wang, H.-F.; Batista, V. S.; Geiger, F. M.. Chem. Phys. Lett. 2017, 683, 199–204. |
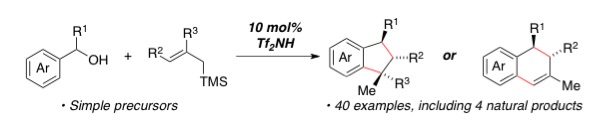
| 57. | Triflimide-Catalyzed Allylsilane Annulations of Benzylic Alcohols for the Divergent Synthesis of Indanes and Tetralins, Reddel, J. C. T.; Wang, W.; Koukounas, K.; Thomson, R. J. Chem. Sci. 2017, 8, 2156–2160. |
| 56. | The Effect of Adding Hydroxyl Functional Groups and Increasing Molar Mass on the Viscosity of Organics Relevant To Secondary Organic Aerosols, Grayson, J. W.; Song, M.; Evoy, E.; Upshur, M. A.; Ebrahimi, M.; Geiger, F. M.; Thomson, R. J.; Bertram, A. K. Atmos. Chem. Phys. 2017, 17, 8509–8523. |

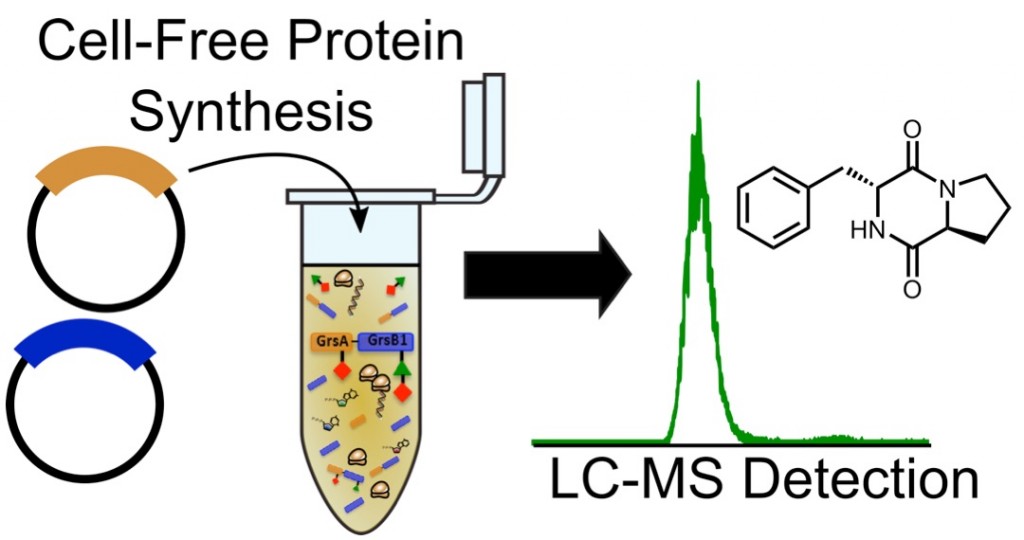
| 55. | In Vitro Reconstruction of Nonribosomal Peptide Biosynthesis Directly from DNA Using Cell-Free Protein Synthesis, Goering, A. W.; Li, J.; McClure, R. A.; Thomson, R. J.; Jewett, M. C.; Kelleher, N. L. ACS Synth. Biol. 2017, 6, 39–44. [link] |
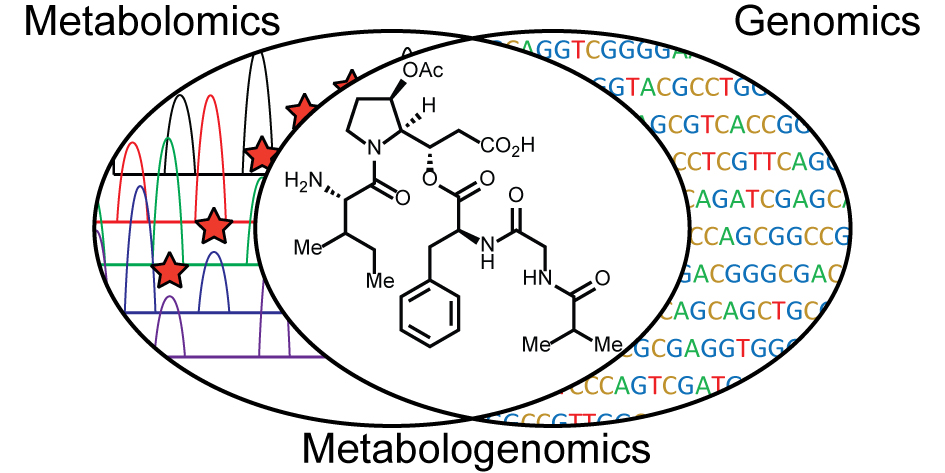
| 54. | Elucidating the Rimosamide-Detoxin Natural Product Families and their Biosynthesis using Metabolite/Gene Cluster Correlations, McClure, R. A.; Goering, A. W.; Ju, K-S.; Baccile, J. A.; Schroeder, F. C.; Metcalf, W. W.; Thomson, R. J.; Kelleher, N. L. ACS Chem. Bio. 2016, 11, 3452–3460. [link] |
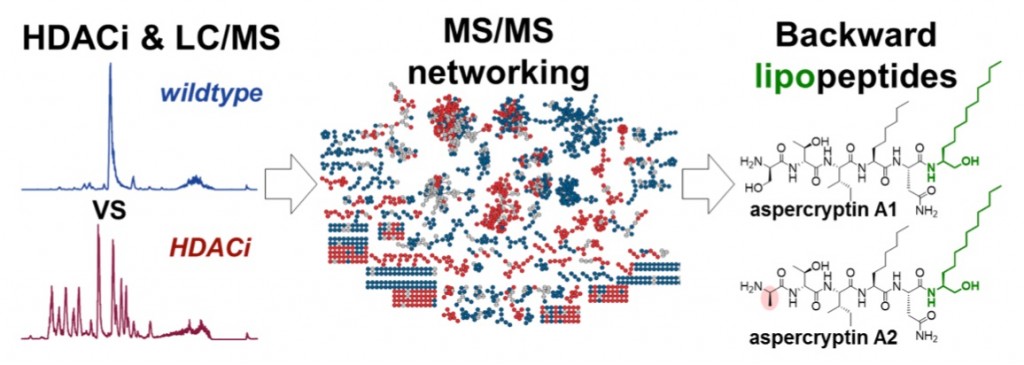
| 53. | New Aspercryptins, Lipopeptides Natural Products Revealed by HDAC Inhibition in Aspergillus nidulans, Henke, M. T.; Soukup, A. A.; Goering, A. W.; McClure, R. A.; Thomson, R. J.; Keller, N. P.; Kelleher, N. L. ACS Chem. Bio. 2016, 11, 2117–2123. [link] |
| 52. | The Structural Elucidation, Chemical Synthesis and Biosynthesis of Prodiginine Alkaloids, Hu, D. X.; Withall, D. M.; Challis, G. L.; Thomson, R. J. Chem. Rev. 2016, 116, 7818–7853. [link] |
| 51. | Sum Frequency Generation Spectroscopy and Molecular Dynamics Simulations Reveal A Rotationally Fluid Adsorption State of α-Pinene on Silica, Ho, J.; Psciuk, B. T.; Chase, H. M.; Rudshteyn, B.; Upshur, M. A.; Fu, L.; Thomson, R. J.; Geiger, F. M.; Batista, V. S. J. Phys. Chem. C. 2016, 120, 12578–12589. [link] |
| 50. | Vibrational Mode Assignment of α-Pinene by Isotope Editing: One Down, Seventy-One to Go, Upshur, M. A.; Chase, H. M.; Strick, B. F.; Ebben, C. J.; Fu, L.; Wang, H.; Thomson, R. J.; Geiger, F. M. J. Phys. Chem. A. 2016, 120, 2684–2690. [link] |
| 49. | Metabologenomics: Correlation of Microbial Gene Clusters with Metabolites Drives Discovery of a Nonribosomal Peptide with an Unusual Amino Acid Monomer, Goering, A. W.; McClure, R. A.; Doroghazi, J. R.; Albright, J. C.; Haverland, N. A.; Zhang, Y.; Ju, K-S.; Thomson, R. J.; Metcalf, W. W.; Kelleher, N.L. ACS Cent. Sci. 2016, 2, 99–108. [link] |
| 48. | Assessment of DFT for Computing Sum Frequency Generation Spectra of an Epoxydiol and a Deuterated Isotopologue at Fused Silica/Vapor Interfaces, Chase, H. M.; Rudshteyn, B.; Psciuk, B. T.; Upshur, M. A.; Strick, B. F.; Thomson, R. J.; Batista, V. S.; Geiger, F. M. J. Phys. Chem. B 2015, 120, 1919–1927. [link] |
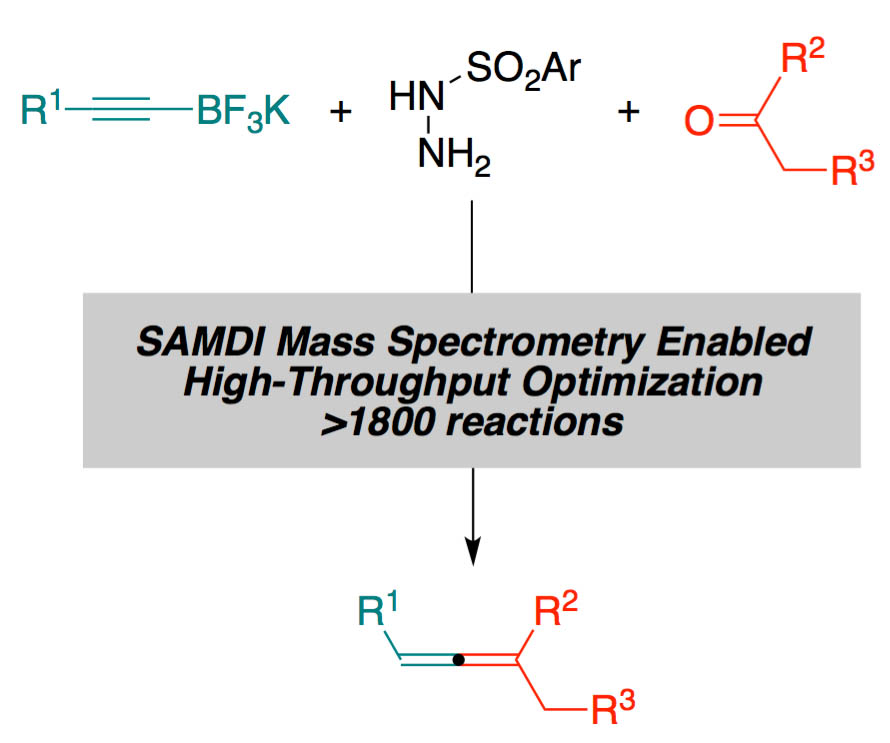
| 47. | SAMDI Mass Spectrometry-Enabled High-Throughput Optimization of a Traceless Petasis Reaction, Diagne, A. B.; Li, S; Perkowski, G. A.; Mrksich, M.; Thomson, R. J. ACS Combi. Sci. 2015, 11, 658–662. [link] |
| 46. | Total Synthesis of the Galbulimima Alkaloids Himandravine and GB17 using Biomimetic Diels–Alder Reactions of Double Diene Precursors, Larson, R. T.; Pemberton, R. P.; Franke, J. M.; Tantillo, D. J.; Thomson, R. J. J. Am. Chem. Soc. 2015, 137, 11197–11204. [link] |
| 45. | Investigations into Apopinene as a Biorenewable Monomer for Ring-Opening Metathesis Polymerization, Strick, B. J.; Delferro, M.; Geiger, F. M.; Thomson, R. J. ACS Sustainable Chem. Eng. 2015, 3, 1278–1281. [link] |
| 44. | Large-Scale Metabolomics Reveals a Complex Response of Aspergillus nidulans to Epigenetic Perturbation, Albright, J. C.; Henke, M. T.; Soukup, A. A.; McClure, R. A.; Thomson, R. J.; Keller, N. P.; Kelleher, N. K. ACS Chem. Biol. 2015, 10, 1535–1541. [link] |
| 43. | Beyond Local Group Modes in Vibrational Sum Frequency Generation, Chase, H. M.; Psciuk, B. T.; Strick, B. F.; Thomson, R. J.; Batista, V. S.; Geiger, F. M. J. Phys. Chem. A. 2015, 119, 3407–3414. [link] |
| 42. | Accurate Lineshapes from Sub-1 cm-1 Resolution Sum Frequency Generation Vibrational Spectroscopy of α-Pinene at Room Temperature, Mifflin, A L.; Velarde, L.; Ho, J.; Psciuk, B. T.; Negre, C. F. A.; Ebben, C. J.; Upshur, M. A.; Lu, Z.; Strick, B. F.; Thomson, R. J.; Batista, V. S.; Wang, H.-F.; Geiger, F. M. J. Phys. Chem. A. 2015, 119, 1292–1302. [link] |
| 41. | On Surface Order and Disorder of α-Pinene-Derived Secondary Organic Material, Shrestha, M.; Zhang, Y.; Upshur, M. A.; Liu, P.; Blair, S. L.; Wang, H.-F.; Nizkorodov, S. A.; Thomson, R. J.; Martin, S. T.; Geiger, F. M. J. Phys. Chem. A. 2015, 119, 4609–4617. (Special Issue in Honor of Prof. Mario Molina) [link] |
| 40. | Enantioselective Synthesis of Metacycloprodigiosin via the “Wasserman Pyrrole”, Vega, M. M; Crain, D. M.; Konkol, L. C.; Thomson, R. J. Tetrahedron Lett. 2015, 56, 3228–3230. (Special Symposium in Print in Memory of Prof. Harry Wasserman) [link] |
| 39. | Uptake of Epoxydiol Isomers Accounts for Half of the Particle-Phase Material Produced from Isoprene Photooxidation via the HO2 pathway, Liu, Y.; Kuwata, M.; Strick, B. F.; Geiger, F. M.; Thomson, R. J.; McKinney, K. A.; Martin, S. T. Env. Sci. Technol. 2015, 49, 250–258. [link] |
| 38. | Enantioselective Total Synthesis of (–)-Maoecrystal V, Zheng, C.; Dubovyk, I.; Lazarski, K. E.; Thomson, R. J. J. Am. Chem. Soc. 2014, 136, 17750–17756. [link] |
| 37. | Climate-Relevant Physical Properties of Molecular Constituents for Isoprene-Derived Secondary Organic Aerosol Material, Upshur, M. A.;Strick, B. F.; McNeil, V. F.; Thomson, R. J.; Geiger, F. M. Atmos. Chem. Phys. 2014, 14, 10731–10740. [link] |
| 36. | Recent Efforts in the Total Synthesis of Isodon Diterpenes, Lazarski, K. E.; Moritz, B. J.; Thomson, R. J. Angew. Chem. Int. Ed. 2014, 53, 10588–10599. [link] |
| 35. | Total Synthesis of Propolisbenzofuran B, Jones, B. T; Avetta, C. T.; Thomson, R. J. Chem. Sci. 2014, 5, 1794–1798. [link] |
| 34. | Towards the Identification of Molecular Constituents Associated with the Surfaces of Isoprene-derived Secondary Organic Aerosol (SOA) Particles, Ebben, C. J.; Strick, B. F.;Upshur, M. A.; Chase, H. M.; Achtyl, J. L.; Thomson, R. J.; Geiger, F. M. Atmos. Chem. Phys. 2014, 14, 2303–2314. [link] |
| 33. | Total Synthesis of the Isodon Diterpene Sculponeatin N, Moritz, B. J.; Mack, D. J.; Tong, L.; Thomson, R. J. Angew. Chem. Int. Ed. 2014, 53, 2988–2991. [link] |
| 32. | Stereocontrolled Syntheses of Tetralone and Naphthyl-type Lignans by a One-Pot Oxidative [3,3] Rearrangement/Friedel–Crafts Arylation, Reddel, J. C. T.; Lutz, K. E.; Diagne, A. B.; Thomson, R. J. Angew. Chem. Int. Ed. 2014, 53, 1395–1398. [link] |
| 31. | Elimination of Butylcycloheptylprodigiosin as a Known Natural Product Inspired by an Evolutionary Hypothesis for Cyclic Prodigiosin Biosynthesis, Jones, B. T.; Hu, D. X.; Savoie, B. M; Thomson, R. J. J. Nat. Prod. 2013, 76, 1937–1945. [link] |
| 30. | Mechanism of Triflimide-Catalyzed [3,3]-Sigmatropic Rearrangements of N-Allylhydrazones—Predictions and Experimental Validation, Gutierrez, O; Strick, B. F.; Thomson, R. J.; Tantillo, D. J. Chem. Sci. 2013, 4, 3997–4003. [link] |

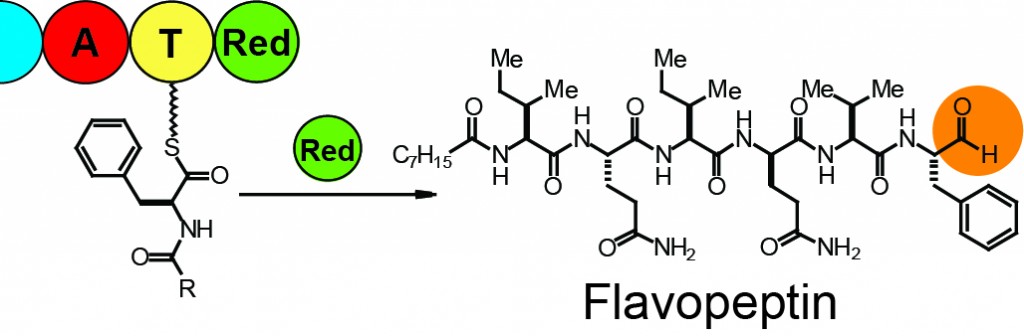
| 29. | Proteomics Guided Discovery of Flavopeptins: Anti-Proliferative Aldehydes Synthesized by a Reductase Domain-Containing Nonribosomal Peptide Synthetase , Chen. Y.; McClure, R. A.; Zheng, Y.; Thomson, R. J.; Kelleher, N. L. J. Am. Chem. Soc. 2013, 135, 10449–10456. [link] |
| 28. | Evaluation of ‘East-to-West’ Ether-Forming Strategies for the Total Synthesis of Maoecrystal V, Lazarski, K. E.; Akpinar, B.; Thomson, R. J. Tetrahedron Lett. 2013, 54, 635-637. [link] |

| 27. | Gobichelin A and B: Mixed-Ligand Siderophores Discovered Using Proteomics, Chen, Y.; Unger, M.; Ntai, I.; McClure, R. A.; Albright, J. C.; Thomson, R. J.; Kelleher, N. L. Med. Chem. Commun. 2013, 4, 233-238. [link] |

| 26. | Oxidative Coupling of Enolates, Enol Silanes, and Enamines: Methods and Natural Product Synthesis, Guo, F.; Clift, M. D.; Thomson, R. J. Eur. J. Org. Chem. 2012, 26, 4881–4896. [link] |

| 25. | Organic Constituents on the Surfaces of Aerosol Particles from Southern Finland, Amazonia, and California Studied by Vibrational Sum Frequency Generation, Ebben, C. J.; Shrestha, M.; Martinez, I. S.; Corrigan, A. L.; Frossard, A. A.; Song, W. W.; Worton, D. R.; Petäjä, T.; Williams, J.; Russell, L. M.; Kulmala, M.; Goldstein, A. H.; Artaxo, P.; Martin, S. T.; Thomson, R. J.; Geiger, F. M. J. Phys. Chem. A, 2012,116, 8271–8290. [link] |

| 24. | Mundal, D. A.; Lutz, K. E.; Thomson, R. J. “A Direct Synthesis of Allenes by a Traceless Petasis Reaction,” J. Am. Chem. Soc. 2012, 134, 5782–5785. [link] |
| 23. | Larson, R. T.; Clift, M. D.; Thomson, R. J. “Total Synthesis of the Galbulimima Alkaloid (–)-GB17,” Angew. Chem. Int. Ed. 2012, 51, 2481–2484. [link] |

| 22. | Konkol, L. C.; Guo, F.; Sarjeant, A. A.; Thomson, R. J. “Enantioselective Total Synthesis and Studies into the Configurational Stability of Bismurrayaquinone A,” Angew. Chem. Int. Ed. 2011, 50, 9931–9934. [link] |

| 21. | Strick, B. F.; Mundal, D. A.; Thomson, R. J. “An Oxidative [2,3]-Sigmatropic Rearrangement of Allylic Hydrazides,” J. Am. Chem. Soc., 2011, 133, 14252–14255. [link] |
![Oxidative[2,3]](https://sites.northwestern.edu/thomson/files/2013/01/Oxidative23-300x109.jpg)
| 20. | Lutz, K. E.; Thomson, R. J. “A Hypervalent Iodide-Initiated Fragment Coupling Cascade of N-Allylhydrazones,” Angew. Chem. Int. Ed. 2011, 50, 4437–4440. [link] |

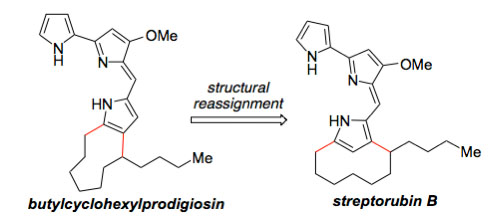
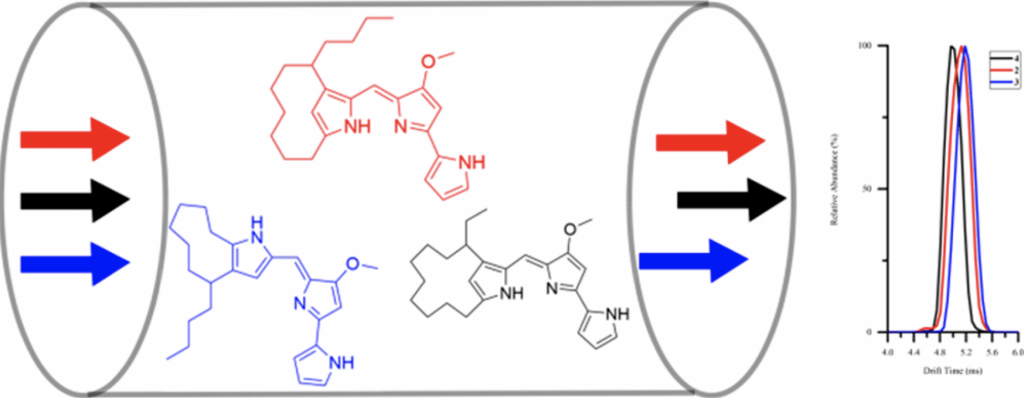
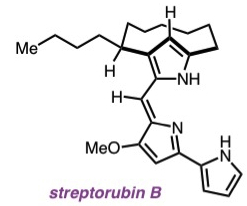
| 19. | Hu, D. X.; Clift, M. D.; Lazarski, K. E.; Thomson, R. J. “Enantioselective Total Synthesis and Confirmation of the Absolute Stereochemistry and Relative Stereochemistry of Streptorubin B,” J. Am. Chem. Soc. 2011, 133, 1799–1804. [link] |
| 18. | Guo, F.; Konkol, L. C.; Thomson, R. J. “Enantioselective Synthesis of Biphenols from 1,4-Diketones by Traceless Central-to-Axial Chirality Exchange,“ J. Am. Chem. Soc. 2011,133, 18–20. [link] |

| 17. | Lazarski, K. E.; Hu, D. X.; Stern, C. L.; Thomson, R. J. “A Synthesis of the Carbocyclic Core of Maoecrystal V,” Org. Lett. 2010, 12, 3010–3013. [link] |

| 16. | Mundal, D. A.; Avetta, Jr. C. T.; Thomson, R. J. “Triflimide Catalyzed Sigmatropic Rearrangement of N-Allylhydrazones as an Example of a Traceless Bond Construction,” Nature Chem. 2010, 2, 294–297. [link] |
![Traceless[3,3]](https://sites.northwestern.edu/thomson/files/2013/01/Traceless33.jpg)
| 15. | Konkol, L. C.; Jones, B. T.; Thomson, R. J. “Oxidative Carbon–Carbon Bond Formation via Allyldimethylsilyl Enol Ethers,” Org. Lett. 2009, 11, 5550–5553. [link] |

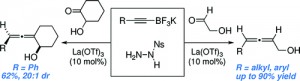
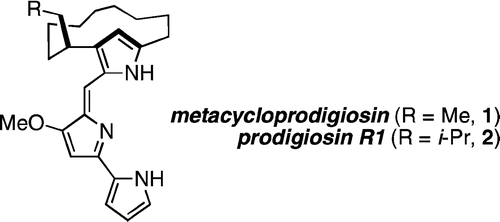
| 14. | Clift, M. D.; Thomson, R. J. “Development of a Merged Conjugate Addition/Oxidative Coupling Sequence. Application to the Enantioselective Total Synthesis of Metacycloprodigiosin and Prodigiosin R1,” J. Am. Chem. Soc. 2009, 131, 14579–14583. [link] |
| 13. | Mundal, D. A.; Lutz, K. E.; Thomson, R. J. “Stereoselective Diene Synthesis from N-Allylhydrazones,” Org. Lett. 2009, 11, 465–468. [link] |
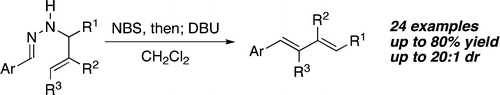
| 12. | Avetta, Jr. C. T.; Konkol, L. C.; Taylor, C. N.; Dugan, K. C.; Stern, C. A.; Thomson, R. J. “Diastereoselective Oxidative Carbon–Carbon Bond Formation via Silyl Bis-enol Ethers,” Org. Lett. 2008, 9, 5621–5624. [link] |

| 11. | Mundal, D. A.; Lee, J. J.; Thomson, R. J. “Tandem Carbon-Carbon and Carbon-Chlorine Bond Formation by Cu(II) Chloride-Promoted [3,3] Sigmatropic Rearrangement of N-Allylhydrazones,” J. Am. Chem. Soc. 2008, 130, 1148–1149. [link] |

| 10. | Clift, M. D.; Taylor, C. N.; Thomson, R. J. “Oxidative Carbon-Carbon Bond Formation via Silyl Bis-enol Ethers: Controlled Cross-coupling for the Synthesis of Quaternary Centers,” Org. Lett. 2007, 9, 4667–4669. [link] |

| 9. | Kim, J.; Thomson, R. J. “Enantioselective Total Synthesis of the Osteoclastogenesis Inhibitor (+)-Symbioimine,” Angew. Chem. Int. Ed. 2007, 46, 3104–3106. [link] |

| 8. | Thomson, R. J. “(S)-4-(Phenylmethyl)-2-thiazolidinethione,” In The Electronic Encyclopedia of Reagents for Organic Synthesis, Wiley, 2007. [link] |
| 7. | Morrison, E.; Chandler, P. M.; Thomson, R. J.; Mander, L. N. “Synthesis and Bioactivity of the Gibberellin, 18-Hydroxy-GA1 (GA132),” Org. Biomol. Chem., 2008, 6, 1416–1424. [link] |
| 6. | Crow, J. R.; Thomson, R. J.; Mander, L. N. “Synthesis and Confirmation of Structure for the Gibberellin GA131 (18-hydroxy-GA4),” Org. Biomol. Chem., 2006, 4, 2532–2544. [link] |
| 5. | Evans, D. A.; Thomson, R. J.; Franco, F. “Ni(II) Tol-BINAP-Catalyzed Enantioselective Michael Additions of b-Ketoesters and Unsaturated N-Acylthiazolidinethiones,” J. Am. Chem. Soc. 2005, 127, 10816–10817. [link] |
| 4. | Evans, D. A.; Thomson, R. J. “Ni(II) Tol-BINAP-Catalyzed Enantioselective Orthoester Alkylations of N-Acylthiazolidinethiones,” J. Am. Chem. Soc. 2005, 127, 10474–10475. [link] |
| 3. | Mander, L. N.; Thomson, R. J. “Total Synthesis of Sordaricin,” J. Org. Chem. 2005, 70, 1654–1670. [link] |
| 2. | Mander, L. N.; Thomson, R. J. “Total Synthesis of Sordaricin,” Org. Lett. 2003, 5, 1321–1324. [link] |
| 1. | Mander, L. N.; Thomson, R. J. “C18-Hydroxylation of Gibberellins,” J. Chem. Soc., Perkin Trans. 1, 2000, 2893–2894. [link] |