BACKGROUND
As our global energy supply expands to meet growing demands and evolves to incorporate a larger fraction of renewable sources, we must adapt our fuel and chemical production industry to effectively utilize, convert, or store these vital resources. Electrochemical catalysts enable a wide array of reactions that can operate at room temperature and pressure and provide a promising approach for buffering and storage of variable renewable energy sources. These reactions are capable of producing a variety of compounds which comprise the foundation of our energy infrastructure and chemical industry with a massive global demand and annual production rates on the order of billions of tons per year. If the feed streams for these processes are composed of waste streams, such as captured CO2, then closed cycles can be formed to reuse critical resources and contribute to net-neutral or even negative emissions.
However, exciting opportunities for electrochemical technologies are limited by our understanding of dynamic catalyst materials that drive these reactions, as well as the complex interplay between bulk reactor properties and the local catalyst environment influencing reaction efficiency and selectivity. Research in the Seitz lab aims to address these challenges.
APPROACH
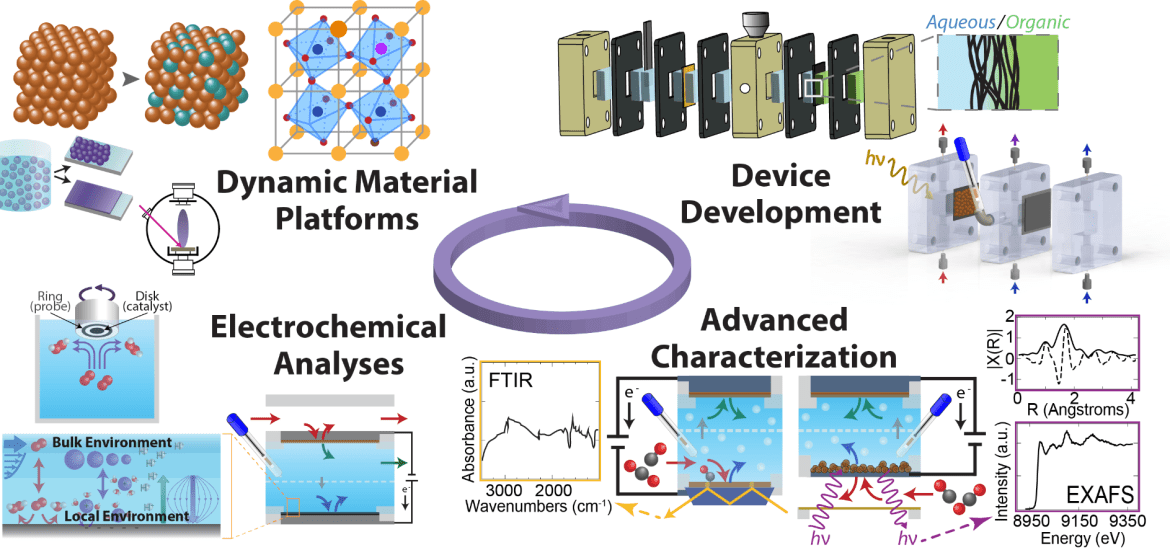
Our approach to developing technologies and improving electrochemical processes is primarily experimental. We use controlled material syntheses to precisely manipulate catalytic active sites for the purpose of understanding reaction mechanisms and defining yet-unresolved structure-reactivity relationships. We also design and fabricate custom reactors that support high current densities, uniquely combine catalytic cycles, or even enable in situ or operando spectroscopic measurements to characterize electronic and geometric structures. Sophisticated experimental platforms and spectroscopic techniques enable us to probe catalyst surface reorganization and deactivation mechanisms as well as complex interfacial structures and surface adsorbates under controlled reaction conditions. Finally, we tune bulk and local reaction environments through reactor design, electrode fabrication, and electrolyte modification to optimize catalyst/electrolyte interfaces and facilitate desired reaction pathways.
Through this work we envision a pathway to improved performance and increased deployment of electrochemical processes for upconversion of waste streams, sustainable production of fuels and chemicals, and enhanced utilization and storage of renewable electricity sources.
RESEARCH AREAS
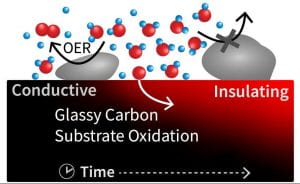
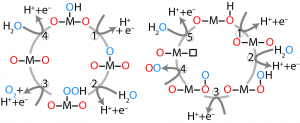
| Electrochemical Oxidation Reactions Determining and controlling source and role of oxygen in selective oxidation mechanisms with relation to catalyst and reaction environment properties. Papers:
|
 |
|
| ~ | ||
| Electrochemical COx Reduction Applying spectroscopic probes of dynamically controlled flow reactors for electroreduction of CO2 with additive chain propagation molecules to investigate C-C coupling mechanisms. Papers: Lu et al. ACS Catal. 2022 |
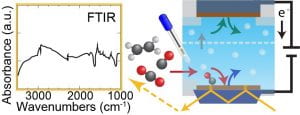 |
|
| ~ | ||
| Complex Oxide/Oxynitride Materials Developing a versatile and controlled material platform based on perovskites and establishing electronic structure effects associated with systematic changes in composition, crystallinity, and strain. Papers: Lu et al. JACS 2022 ~ Edgington et al. JACS 2021 |
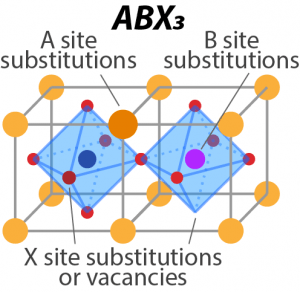 |
|
| ~ | ||
| Electrochemical Double Layer Developing and integrating experimental probes and multiscale models of species and potential gradients between bulk reactor environment and local catalyst environment. Papers: Ruggiero et al. J. Phys. Chem. C 2023 ~ Ruggiero et al. J. Catal. 2022 |
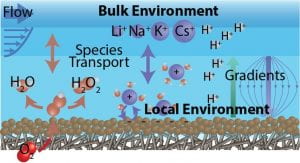 |
|
| ~ | ||
| Electrode Deactivation Mechanisms Probing dynamic catalyst surfaces and electrode supports during reaction conditions and elucidating driving forces and mechanisms for degradation. Papers: Li et al. ACS Energy Fuels 2023 ~ Edgington et al. ACS Appl. Energy Mater. 2022 ~ Park et al. Adv. Func. Mater. 2022 ~ Edgington et al. JACS 2021 |
|
THREE MINUTE THESES
Can you describe your thesis research in three minutes with no/limited jargon? Members of the Seitz Lab can! Check out our video submissions (two of which won second and third place!) below for the Three Minute Thesis Competition as founded by The University of Queensland and hosted by the Center for Engineering Sustainability and Resilience at Northwestern University!
- “Anion Tuning of Perovskite Materials” – Matthew Sweers
- “Electrifying Change: Improving Catalysts for Electrochemical Water Splitting” – Jane Edgington
- “Sustainable Production of Hydrogen Peroxide Using Renewable Electricity” – Brianna Ruggiero
- “Catalyst Corrosion for Water Electrolysis” – Ruihan Li
- “Catalyzing a Sustainable Future with Electrochemistry” – Adrien Deberghes