My latest study is now revealed! This one is a new technique to measure specific surface area for heretofore very challenging systems under conventional techniques. It makes use of how absolutely robust trimethylaluminum (TMA) is for atomic layer deposition. It’s the quintessential precursor and behaves as one would expect for an ALD process, but as my first study showed, that is definitely not the case for all precursors (false advertising!). TMA and water can be used to recreate the surface details of a small, nanorough surface. Then the Al can be quantified analytically with ICP, down to very small amounts with high sensitivity. This should allow electrode researchers to quantify the all-important surface area metric without generating bulk amounts of material (exceeding 1 square meter), particularly in the high temperature catalysis community since our high surface areas are still low compared to those of the MOF community, for example.
G02-1671 – Atomic Layer Deposition for Surface Area Characterization of Porous Solid Oxide Fuel Cell Electrodes and Beyond
Abstract
Conventional surface area determination techniques are inadequate for calculating the surface areas of porous solids with absolute totals less than ~1 m2, but with nm-scale feature sizes, such as state-of-the-art, lab-scale solid oxide fuel cell electrodes. Meaningful contextualization of their electrochemical performance requires knowing system surface area, but the two predominant classes of techniques, tomography and gas adsorption theory, either lack the resolution or require prohibitive amounts of material. Presented here is a new method for the accurate determination of absolute surface areas on the order of 1-1000 cm2 with precision ±0.3 cm2 utilizing atomic layer deposition (ALD) of commonly available trimethylaluminum (TMA) and water vapor to conformally deposit alumina over internal features. The area of alumina can then be quantified using plasma spectroscopy and the known nm-scale thickness of the layer. As a model system, scaffolds of La0.8Sr0.2MnO3-δ/Ce0.9Gd0.1O2-δ (LSM/GDC) of 2.6 m2/g were measured under the new technique and compared against the B.E.T. method, with remarkably similar results obtained, but with ~1000 times less mass needed for the experiment. Under the modest ALD reactor soak times used (~10 s), and with conservative precursor pulse durations, the penetration depth of the coating was found to be ~50 µm, exceeding the requirement for the electrodes under study. To demonstrate the technique’s capabilities further, the LSM/GDC scaffolds were infiltrated with PrOx nanoparticles of ~50 nm, and the increase in surface area (+ ~25%) and enhanced electrochemical performance were measured. After annealing for 1000 hours at 700°C, both were again measured, and the surface area was found to have reverted to non-infiltrated levels, despite the infiltrated cells maintaining their higher performance. The technique, due to its ease versus other surface area determination methods and its pairing with an established analytical chemistry method, enables a useful combined chemical/morphological approach to the characterization of next-generation solid oxide cell electrodes, and may find other uses among nanostructured yet low absolute surface area systems in the broad field of heterogeneous catalysis.
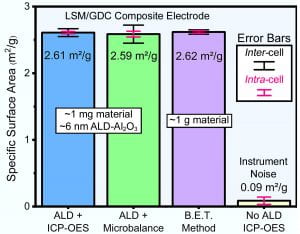
Figure 1. Measured specific surface areas for La0.8Sr0.2MnO3-δ/Ce0.9Gd0.1O2-δ (LSM/GDC) scaffolds and the ALD Al2O3-based technique, in lab-scale mg quantities, versus the B.E.T. method result for gram-scale LSM/GDC flakes. Shown is ALD-derived surface area quantified both via inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Blue) and gravimetrically (Green), with the ICP-OES offering comparable accuracy and precision to the B.E.T. (Purple). Error bars reflect repetition of the same sample (intra-cell) and among different samples (inter-cell).