About Our Lab
The Kiyokawa lab investigates the molecular, biological and translational properties of posttranlational modifications of cellular proteins, especially ubiquitination. Our long-term goal is to develop novel therapeutic strategies to target protein ubiquitination in patients with breast and other types of cancers, and neurodevelopmental and neurodegenerative disorders.
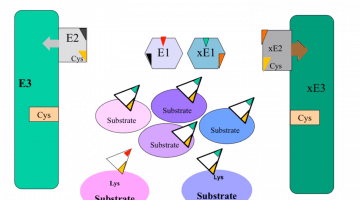
Over two decades our lab has been studying mechanisms of cell proliferation, differentiation and senescence, and our current effort focuses on several E3 ubiquitin ligases including E6AP/UBE3A, CHIP/STUB1 and Parkin, as well as on the two E1 ubiquitin activating enzymes UBA1 and UBA6. We are especially interested in how those ubiquitination enzymes are genetically or epigenetically altered in human diseases, which ubiquitination substrates are affected as downstream pathogenic drivers, and how we could pharmacologically target the changes.
We use diverse experimental model systems such as genetically modified mice with knockout or transgenic overexpression of oncogenes and tumor suppressor genes (e.g., Cdk4, Cdc25a, Cdkn1b), induced pluripotent stem cells (iPSCs) combined with CRISPR/Cas9 gene editing, and a novel proteomic screening platform called Orthogonal Ubiquitin Transfer (OUT), and small molecule screens for ubiquitination modifiers.
Overview
Research

Members

Join the Lab: Postdoctoral Position Available
