
Expertise
- Radiogenic Isotope Geochemistry
- Low-Temperature Aqueous Geochemistry
- Calcium Isotopes
- Strontium Isotopes
- Carbonate Geochemistry
- Earth Surface Processes
- Chemical Weathering
- Long-Term Carbon Cycle
- Paleoceanography
- Paleoclimatology
- Carbon Dioxide Removal
My laboratory specializes in the high-precision analysis of stable calcium and strontium isotope abundance variations (δ44/40Ca and δ88/86Sr) by thermal ionization mass spectrometry (TIMS). Group members apply these measurements to diverse topics ranging from paleoclimate reconstructions, mass extinctions, and basalt weathering to biomineralization, dinosaur trophic levels, and osteoporosis. Many projects have implications for nascent carbon dioxide removal strategies designed to mitigate future climate warming, including ocean alkalinity enhancement, enhanced rock weathering, and carbon mineralization at elevated temperatures.
Recent Highlights
Enhanced Rock Weathering
I recently received a $5M Negative Carbon Shot award from the Department of Energy to investigate Enhanced Rock Weathering (ERW) as a decarbonization strategy for limiting future climate warming. The research will focus on enabling carbon drawdown in the US Midwest through enhanced carbonate mineral weathering. Key partners and co-investigators include Silicate Carbon, Yardstick, Prof. Frank McDermott in the School of Earth Science at University College Dublin, Prof. Louise Egerton-Warburton at the Chicago Botanic Garden, and Prof. Brad Sageman in the Department of Earth and Planetary Sciences at Northwestern University.
Natural rock weathering transforms CO2 to HCO3–, a dissolved form of carbon that is largely stable in solution over human timescales. ERW is a geoengineering strategy that seeks to accelerate this process by applying pulverized minerals and rocks to agricultural soils. A multidisciplinary approach is crucial for successfully implementing ERW, but ERW remains in its infancy. Basic science investigations are needed to develop robust, transparent, and reliable protocols for verifying CO2 removal (CDR) by ERW.
The chemical weathering of Ca- and Mg-bearing silicate rocks removes atmospheric CO2 and regulates climate over geologic timescales. Most ERW strategies focus on mafic silicate rocks, such as basalt, which are widely expected to dissolve faster than felsic rocks, such as granite. The “relative mineral weathering stability paradigm” traces to an early study conducted nearly a century ago. More recent studies have interpreted high HCO3– fluxes from basalt-draining rivers as direct evidence for the rapid weatherability of basalt.

However, high-precision Ca isotope measurements applied to Icelandic rivers demonstrate that basalt weathering rates are slower than previously realized. As shown in the figure above, most riverine Ca2+ (and thus a significant fraction of the balancing HCO3– by extension) derives from the chemical weathering of Iceland spar and other forms of hydrothermal calcite that compose a minor proportion of the bedrock. My students and I have documented this phenomenon in other regions throughout the globe, including Greenland, the north slope of Alaska, the Himalaya Mountains of northern Pakistan, and the New Zealand Southern Alps.
The result is consistent with long-standing knowledge that calcite dissolves orders of magnitude faster than both mafic and felsic silicate minerals. While carbonate weathering does not affect atmospheric CO2 levels over geologic timescales, half of the HCO3– produced represents a carbon sink over human timescales. These and other properties make limestone an attractive feedstock candidate for emerging ERW strategies. The Negative Carbon Shot study will field test the CDR efficacy of limestone, basalt, and other geologic materials. The research will also develop naturally occurring isotopes of Ca, Sr, and other elements as novel tools for measurement, reporting, and verification criteria surrounding CDR estimates.

Meanwhile, with generous support from the Paula M. Trienens Institute for Sustainability and Energy, we are conducting mesocosm experiments at the Chicago Botanic Garden to test the CDR efficacy of basaltic amendments applied to typical midwestern agricultural soils. We are examining the effects of temperature, crop type, and other variables using an array of seventy-three mesocosms. Data and insights gleaned from these experiments will work synergistically with the Negative Carbon Shot award to advance understanding of ERW.
Basalt Weathering
I maintain an active interest in the fundamental nature of basalt weathering. The figure below shows δ44/40Ca values for Icelandic zeolites, hydrothermal calcite, and hydrothermal waters, including natural waters and those produced during field-scale pilot carbon capture sequestration experiments (CarbFix). The δ44/40Ca values strongly correlate with average Ca-O bond lengths, apart from one zeolite. The data provide evidence for equilibrium isotope partitioning and have several implications. First, the Ca isotope geochemistry of zeolites can be developed into a novel tool for investigating low-grade basalt metamorphism. Second, hydrothermal waters have high δ44/40Ca values because zeolites (especially heulandite) sequester lighter Ca isotopes. Third, the similar δ44/40Ca values between hydrothermal water and calcite provide evidence that calcite precipitates in isotopic equilibrium with hydrothermal water (i.e., the equilibrium fractionation factor for the calcite-water system is 0‰). Fourth, Icelandic rivers have higher δ44/40Ca values than bulk basalt. As noted above, the chemical weathering of isotopically heavy calcite readily explains this pattern. All data combined illustrate the potential of using Ca isotopes to track the efficacy of carbon mineralization in basalt at elevated temperatures.

Deep Time Ocean Acidification Events
Several group members have worked to develop δ44/40Ca and δ88/86Sr as proxies for deep time ocean acidification events. For decades, Earth Scientists have hypothesized that CO2 emissions during Large Igneous Province (LIP) eruptions should suppress biocalcification by acidifying seawater, decreasing carbonate anion concentrations, and lowering carbonate mineral saturation states.
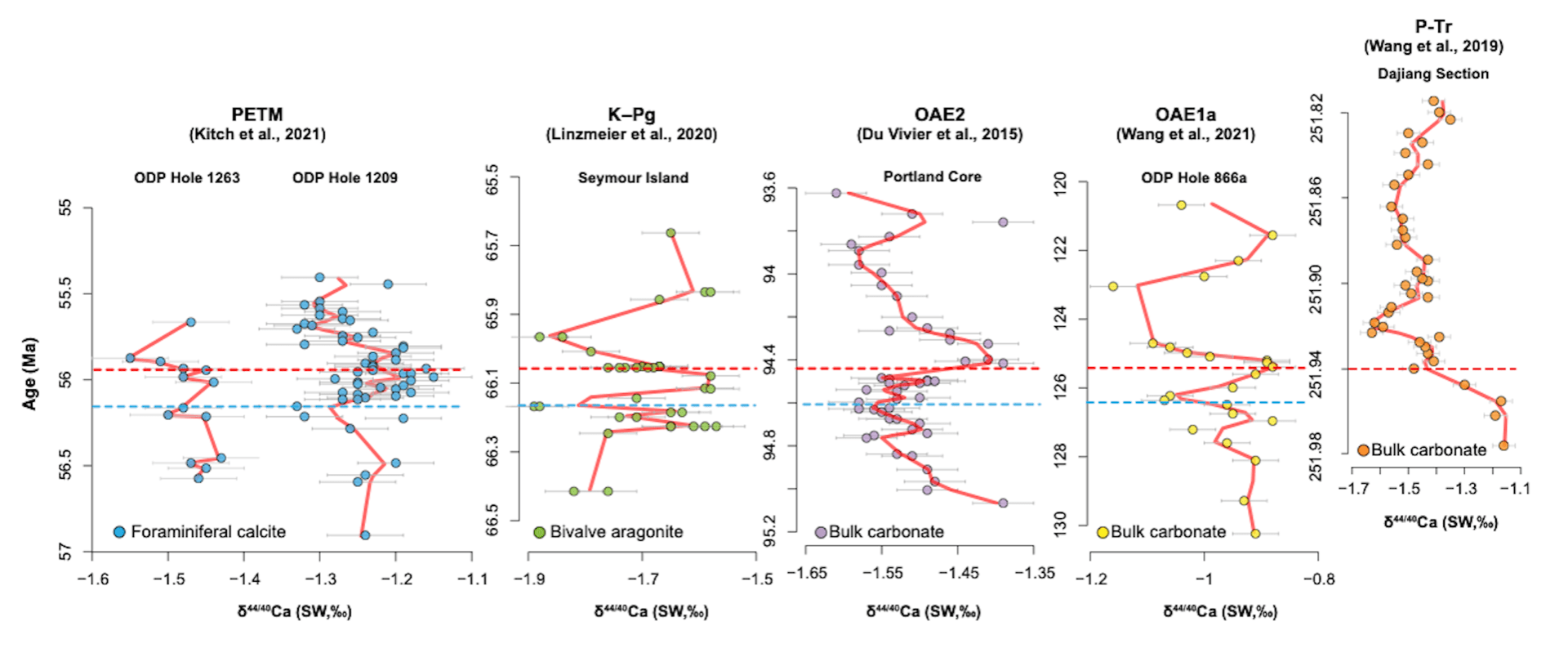
The figure above shows δ44/40Ca records generated for five candidate ocean acidification events, including the the Permian-Triassic boundary (P-Tr, ~252 Ma, Siberian Traps LIP), Ocean Anoxic Event 1a (OAE1a, ~120 Ma, Ontong Java Plateau LIP), Ocean Anoxic Event 2 (OAE2, ~94 Ma, Caribbean and/or High Arctic LIPs), the Cretaceous-Paleogene boundary (K-Pg, ~66 Ma, Deccan Traps LIP), and the Paleocene-Eocene Thermal Maximum (PETM, ~56 Ma, North Atlantic Igneous Province LIP).
Striking patterns emerge when the records are aligned relative to their canonically defined major boundaries (dashed red lines denote δ13C excursions for the P-Tr, OAE1a, OAE2, and PETM records and the Ir layer for the K-Pg record). The P-Tr record exhibits a large and rapid negative excursion. The other four records show similar patterns, including both negative and positive shifts before the boundaries, negative excursions after the boundaries, and positive recoveries. For the K-Pg record, the small negative excursion preceding the Ir layer corresponds to a local minor extinction (dashed blue line). The OAE1a, OAE2, and PETM records also show analytically resolvable pre-event negative shifts that align with the one observed for the K-Pg record.
The apparent similarities are notable given that the records represent different depositional settings (epeiric sea, carbonate platform, slope margin, and deep sea), archives (bulk carbonate sediments, microfossils, and macrofossils), mineralogies (calcite and aragonite), and event durations (~0.2 to 1 Myr). The data appear most consistent with preservation of primary kinetic isotope effects (precipitation rate-dependent shifts in Ca isotope fractionation) and provide less support for alternative hypotheses, such as early diagenesis and input-output flux imbalances. Trends toward higher δ44/40Ca values are consistent with slower carbonate precipitation rates, whereas trends toward lower δ44/40Ca values are consistent with faster carbonate precipitation rates. All data combined point to “biological compensation” as an underappreciated dampening mechanism to ocean acidification.
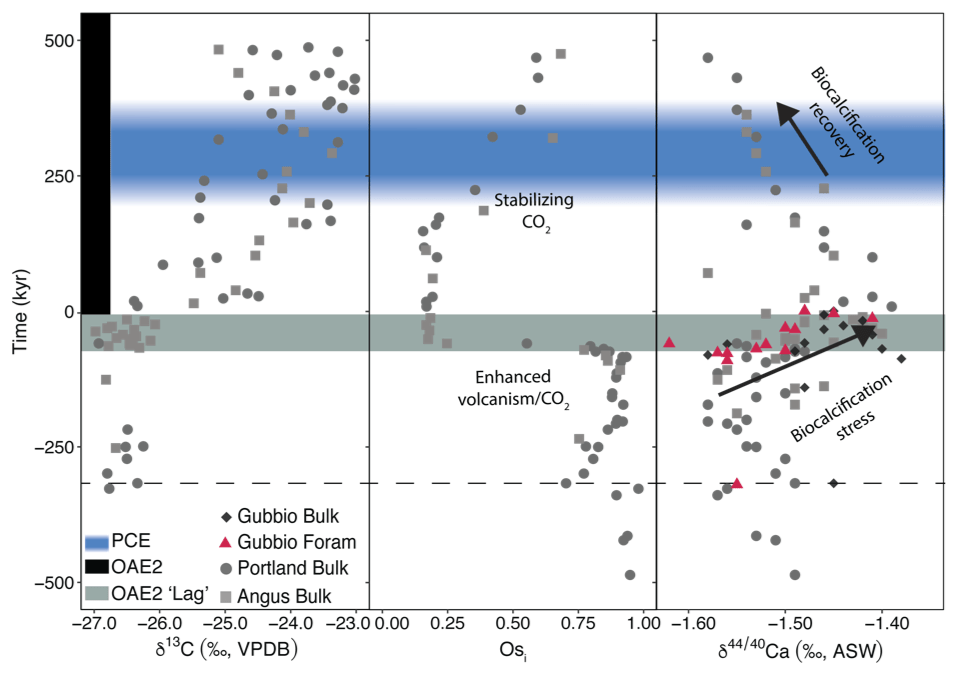
A recent study of OAE2 shows that δ44/40Ca values measured in different archives (bulk carbonate and foraminifera) from globally distributed locations (Western Interior Seaway and Tethys) collectively define a similar pattern that correlates with fluctuations in the Os isotope (Osi) composition seawater, a well-established tracer for LIP eruptions. A positive Ca isotope excursion that begins before the onset OAE2 coincides with a negative Osi excursion. The combined signals are consistent with ocean acidification due to CO2 inputs from LIP volcanism.

Prior to the onset of OAE2, foraminiferal (R. cushmani) δ44/40Ca values increase as shells become smaller and malformed. Progressively decreased calcification rates provide the simplest explanation for this pattern.
Interestingly, an enigmatic period of climate cooling in the aftermath of OAE2 (the Plenus Cold Event) coincides with Ca isotope evidence for ocean alkalinization deriving from suppressed biocalcification. The data highlight OAE2 as a geological analog for artificial ocean alkalinization, a leading decarbonization strategy.

Further insight comes from comparing δ44/40Ca and δ88/86Sr values (the “δ44/40Ca-δ88/86Sr multi-proxy”). The figure above shows δ44/40Ca and δ88/86Sr data for the P-Tr and OAE1a. Values spanning the P-Tr boundary do not define a clear pattern, whereas data for OAE1a define a line with a slope of 0.19, which is the value predicted from kinetic mass-fractionation theory and recovered in bench-scale calcite precipitation experiments. Detection of kinetic isotope effects in the Phanerozoic rock record has numerous implications for understanding relationships between LIP eruptions and biocalcification feedbacks.

Numerical modeling cannot resolve the origin of Ca isotope stratigraphic signals, as leading primary precipitation and secondary alteration models predict identical patterns for measured data.

While claims are made that diagenesis can simulate primary kinetic isotope effects, diagenetic theory less satisfactorily explains why nominally highly altered samples yield identical stratigraphic signals as pristine samples. The OAE1a stratigraphic record represents bulk calcite sediments, while the K-Pg record represents aragonitic macrofossils.

Linear regression of the K-Pg dataset pinpoints the aragonite Ca isotope equilibrium fractionation factor measured in laboratory precipitation experiments. This provides more evidence that the K-Pg Ca isotope record is the byproduct of primary kinetic isotope effects.

Instances of diagenesis that have been documented via empirical inquiry demonstrate that alteration introduces noise, which is superimposed on the main stratigraphic signals. For OAE2 (Portland #1 Core), a few limestone samples with anomalously low δ13C values have slightly elevated δ44/40Ca values. These data are consistent with a small amount of carbonate dissolution/re-precipitation driven by the microbial respiration of organic matter. For the K-Pg boundary record, alteration of molluscan aragonite templates more aragonite, leading to shell material with lower δ44/40Ca values and Sr/Ca ratios, not higher as diagenetic models assume.

As shown in the figure above, lab members have also detected the kinetic slope in Neoproterozoic carbonates, including a cap dolostone deposited after the Marinoan Snowball Earth Event and limestones deposited before the Sturtian Snowball Earth Event. As with Phanerozoic carbonates (e.g., P-Tr), not all Neoproterozoic carbonates yield the kinetic slope. For example, another Marinoan cap dolostone shows a negative correlation between δ44/40Ca and δ88/86Sr. Deviations from an ideal mass-fractionation line may reflect several factors. Like the P-Tr carbonates, the example illustrated above for the Ombaatjie dolostone is consistent with a shift in the isotopic composition of seawater. Most likely, these shifts are local rather than global, as coastal carbonates are sensitive to inputs from rivers and submarine groundwater discharge. Detection of the kinetic slope in multiple forms of carbonate from different time periods provides impetus to develop δ44/40Ca and δ88/86Sr as sensitive proxies for pCO2 and carbonate mineral saturation states.

For the Arbeitsgenot cap dolostone, diagenetic theory insufficiently reconciles why samples theoretically displaying the least altered (lowest) δ44/40Ca and δ88/86Sr values display the most altered (highest) 87Sr/86Sr ratios. Nor can diagenesis easily reconcile why the Arbeitsgenot cap dolostone displays the kinetic slope, while the Ombaatjie cap dolostone does not.
A kinetic interpretation explains all available evidence. The Arbeitsgenot dolostone or its precursor phase began forming near isotopic equilibrium with a meltwater-dominated mixture and precipitation rates increased up-section. Samples exhibiting faster precipitation rates display progressively higher 87Sr/86Sr ratios. This pattern is consistent with long-held assertions that alkalinity from a rapid deglacial weathering pulse forced cap carbonate deposition. The negative slope observed for the Ombaatjie cap dolostone suggests that the seawater-meltwater mixing ratio became increasingly seawater-dominated up-section. Nonetheless, the distance between the datapoints and the theoretical mixing domain increases up-section, which implies that precipitation rates increased up-section. Here too, faster precipitation rates correlate with higher 87Sr/86Sr ratios, which further points to a causal connection between enhanced chemical weathering and cap carbonate formation.
Taken together, all studies demonstrate that diagenetic alteration of Ca and Sr isotopes in marine carbonate minerals is highly rock-buffered. We find that Earth’s carbonate rock record is a high-fidelity archive of primary Ca and Sr isotope abundance variations mostly deriving from kinetic mass-dependent fractionation.