
Expertise
Radiogenic Isotope Geochemistry, Low-Temperature Aqueous Geochemistry, Carbonate Geochemistry, Earth Surface Processes, Chemical Weathering, Long-Term Carbon Cycle, Paleoceanography, Calcium Isotopes, Strontium Isotopes
My laboratory specializes in the high-precision analysis of stable calcium and strontium isotope abundance variations (δ44/40Ca and δ88/86Sr) by thermal ionization mass spectrometry (TIMS). Group members apply these measurements to diverse problems ranging from paleoclimate reconstructions to basalt weathering. Many projects have implications for emerging decarbonization technologies designed to mitigate the anthropogenic climate crisis, including ocean alkalinity enhancement, enhanced rock weathering, and carbon mineralization at elevated temperatures.
Recent Highlights
Several group members have worked to develop δ44/40Ca and δ88/86Sr as proxies for ocean acidification events in deep time. For decades, Earth Scientists have hypothesized that CO2 emissions during Large Igneous Province (LIP) eruptions should suppress biocalcification by acidifying seawater, decreasing carbonate anion concentrations, and lowering carbonate mineral saturation states.
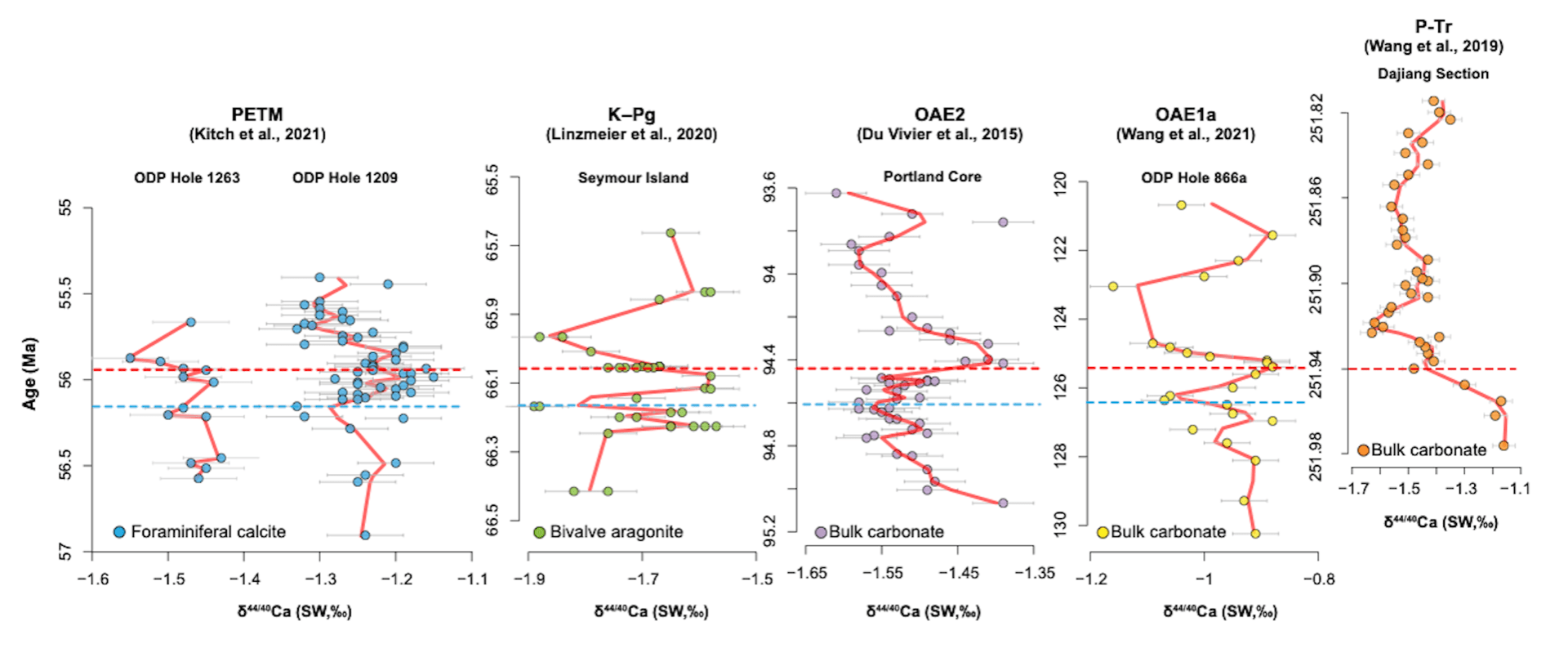
The figure above shows δ44/40Ca records generated for five candidate ocean acidification events, including the the Permian-Triassic boundary (P-Tr, ~252 Ma, Siberian Traps LIP), Ocean Anoxic Event 1a (OAE1a, ~120 Ma, Ontong Java Plateau LIP), Ocean Anoxic Event 2 (OAE2, ~94 Ma, Caribbean and/or High Arctic LIPs), the Cretaceous-Paleogene boundary (K-Pg, ~66 Ma, Deccan Traps LIP), and the Paleocene-Eocene Thermal Maximum (PETM, ~56 Ma, North Atlantic Igneous Province LIP).
Striking patterns emerge when the records are aligned relative to their canonically defined major boundaries (dashed red lines denote δ13C excursions for the P-Tr, OAE1a, OAE2, and PETM records and the Ir layer for the K-Pg record). The P-Tr record exhibits a large and rapid negative excursion. The other four records show similar patterns, including both negative and positive shifts before the boundaries, negative excursions after the boundaries, and positive recoveries. For the K-Pg record, the small negative excursion preceding the Ir layer corresponds to a local minor extinction (dashed blue line). The OAE1a, OAE2, and PETM records also show analytically resolvable pre-event negative shifts that align with the one observed for the K-Pg record.
The apparent similarities are notable given that the records represent different depositional settings (epeiric sea, carbonate platform, slope margin, and deep sea), archives (bulk carbonate sediments, microfossils, and macrofossils), mineralogies (calcite and aragonite), and event durations (~0.2 to 1 Myr). The data appear most consistent with preservation of primary kinetic isotope effects (precipitation rate-dependent shifts in Ca isotope fractionation) and provide less support for alternative hypotheses, such as early diagenesis and input-output flux imbalances. Trends toward higher δ44/40Ca values are consistent with slower carbonate precipitation rates, whereas trends toward lower δ44/40Ca values are consistent with faster carbonate precipitation rates. All data combined point to “biological compensation” as an underappreciated dampening mechanism to ocean acidification.
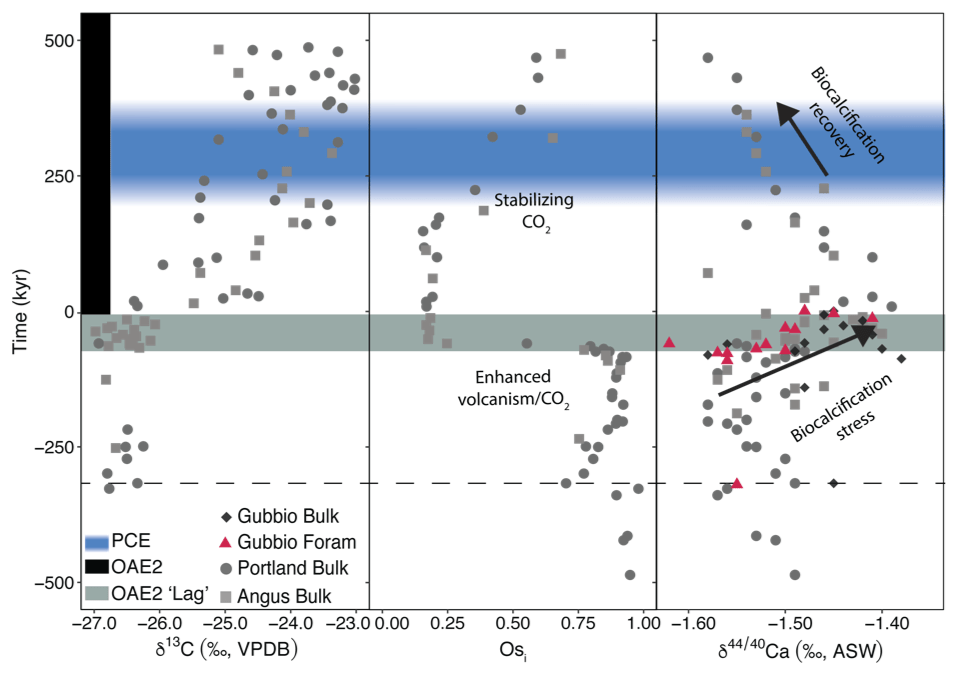
A recent study of OAE2 shows that δ44/40Ca values measured in different archives (bulk carbonate and foraminifera) from globally distributed locations (Western Interior Seaway and Tethys) collectively define a similar pattern that correlates with fluctuations in the Os isotope (Osi) composition seawater, a well-established tracer for LIP eruptions. A positive Ca isotope excursion that begins before the onset OAE2 coincides with a negative Osi excursion. The combined signals are consistent with ocean acidification due to CO2 inputs from LIP volcanism. Prior to the onset of OAE2, foraminiferal (R. cushmani) δ44/40Ca values increase as shells become smaller and malformed. These data suggest ocean acidification decreased biocalcification rates. Interestingly, an enigmatic period of climate cooling in the aftermath of OAE2 (the Plenus Cold Event) coincides with Ca isotope evidence for ocean alkalinization deriving from suppressed biocalcification. The data highlight OAE2 as a geological analog for artificial ocean alkalinization, a leading decarbonization strategy.

Further insight comes from comparing δ44/40Ca and δ88/86Sr values (the “δ44/40Ca-δ88/86Sr multi-proxy”). The figure above shows δ44/40Ca and δ88/86Sr data for the P-Tr and OAE1a. Values spanning the P-Tr boundary do not define a clear pattern, whereas data for OAE1a define a line with a slope of 0.19, which is the value predicted from kinetic mass-fractionation theory and recovered in bench-scale calcite precipitation experiments. Early diagenesis (an isotopic mixing mechanism) cannot simulate the “kinetic slope” because primary and secondary carbonate have different Sr/Ca ratios. Detection of kinetic isotope effects in the Phanerozoic rock record has numerous implications for understanding relationships between LIP eruptions and biocalcification feedbacks.

As shown in the figure above, lab members have also detected the “kinetic slope” in Neoproterozoic carbonates, including a “cap dolostone” deposited after the Marinoan Snowball Earth Event and limestones deposited before the Sturtian Snowball Earth Event. As with Phanerozoic carbonates (e.g., P-Tr), not all Neoproterozoic carbonates yield the “kinetic slope.” For example, another Marinoan “cap dolostone” shows a negative correlation between δ44/40Ca and δ88/86Sr. Deviations from an ideal mass-fractionation line may reflect several factors. Like the P-Tr carbonates, the example illustrated above for the Ombaatjie dolostone is consistent with a shift in the isotopic composition of seawater. Most likely, these shifts are local rather than global, as coastal carbonates are sensitive to inputs from rivers and submarine groundwater discharge. Detection of the “kinetic slope” in multiple forms of carbonate from different time periods provides impetus to develop δ44/40Ca and δ88/86Sr as sensitive proxies for pCO2 and carbonate mineral saturation states.

Entirely different efforts have focused on using Ca isotopes to trace basalt weathering. The figure above shows δ44/40Ca values for Icelandic zeolites, hydrothermal calcite, and hydrothermal waters, including natural waters and those produced during pilot carbon capture sequestration experiments (CarbFix). The δ44/40Ca values strongly correlate with average Ca-O bond lengths, with the exception of one zeolite. The data provide evidence for equilibrium isotope partitioning and have several implications. First, the Ca isotope geochemistry of zeolites can be developed into a novel tool for investigating low-grade basalt metamorphism because equilibrium fractionation factors are temperature-sensitive. Second, hydrothermal waters have high δ44/40Ca values because zeolites (especially heulandite) sequester lighter Ca isotopes. Third, the similar δ44/40Ca values between hydrothermal water and calcite provide evidence that calcite precipitates in isotopic equilibrium with hydrothermal water (i.e., the equilibrium fractionation factor for the calcite-water system is 0‰). Fourth, Icelandic rivers have higher δ44/40Ca values than bulk basalt. Both groundwater discharge and the chemical weathering of isotopically heavy calcite readily explain this pattern. Finally, all data combined illustrate the potential of using Ca isotopes to track the efficacy leading decarbonization strategies, such as enhanced rock weathering and carbon mineralization at elevated temperatures.