Driving Research Forward
We are a diverse team of scientists seeking to advance fundamental knowledge and impact society.
Complex Synthesis ›
Our Complex Synthesis platform is where strategic planning meets practical execution. The ability to design and conduct syntheses of architecturally complex and biologically active small molecules is invaluable for both scientific and personal advancement.
Catalysis ›
Our Catalysis platform seeks to discover, develop, and apply new catalytic methods for the efficient and selective synthesis of biomedically relevant molecules.
Translational Chemistry ›
Our dynamic Translational Chemistry platform focuses on expanding the applications of natural product-inspired compounds and using these unique scaffolds to target biologically relevant systems.
Latest Publications
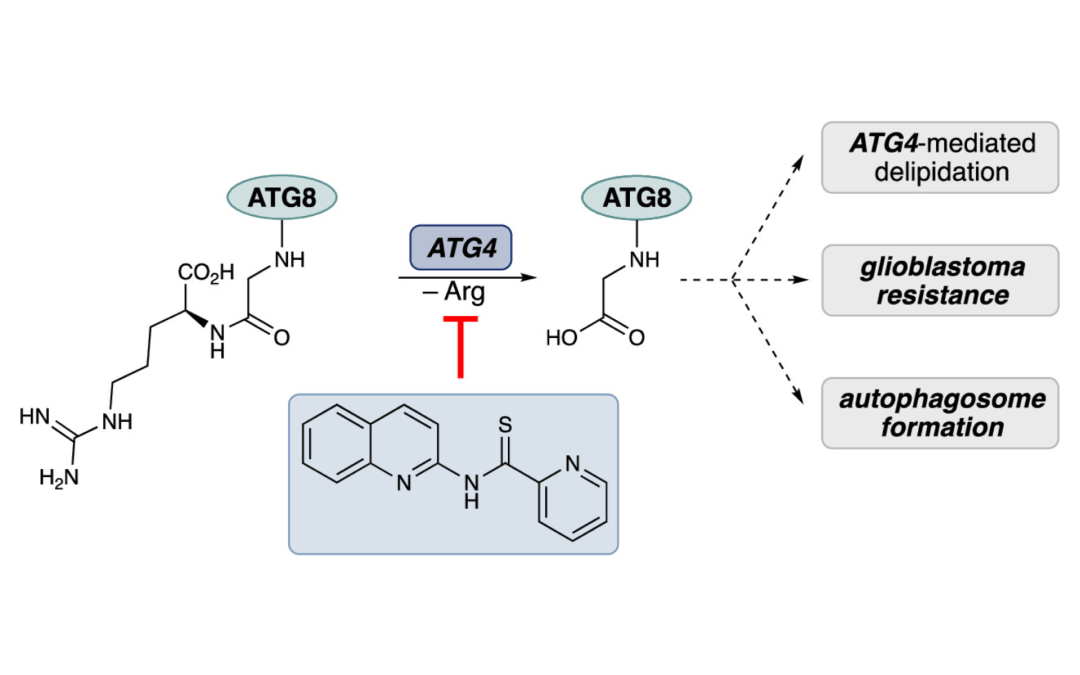
Synthesis and Structural Optimization of ATG4B Inhibitors for the Attenuation of Autophagy in Glioblastoma
Kim. D. R.; Orr, M. J.; Yu, X.; Munshi, H. H.; Wang, A.; Trudeau, C.; Kwong, A. J.; Cheng, S.-Y.; Scheidt, K. A.* ACS Med. Chem. Lett. 2024, 15, 258-264.
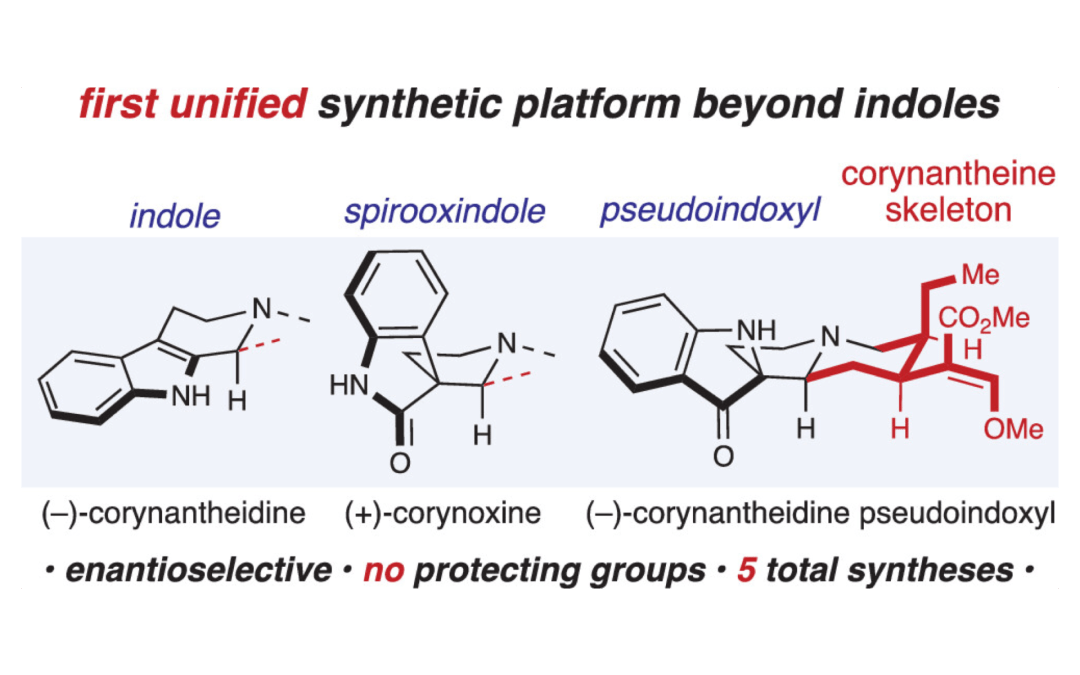
A Platform for the Synthesis of Corynantheine-Type Corynanthe Alkaloids
Nam, Y.; Tam, A. T.; Miller, E. R.; Scheidt, K. A.* J. Am. Chem. Soc. 2024, 146, 118-124.
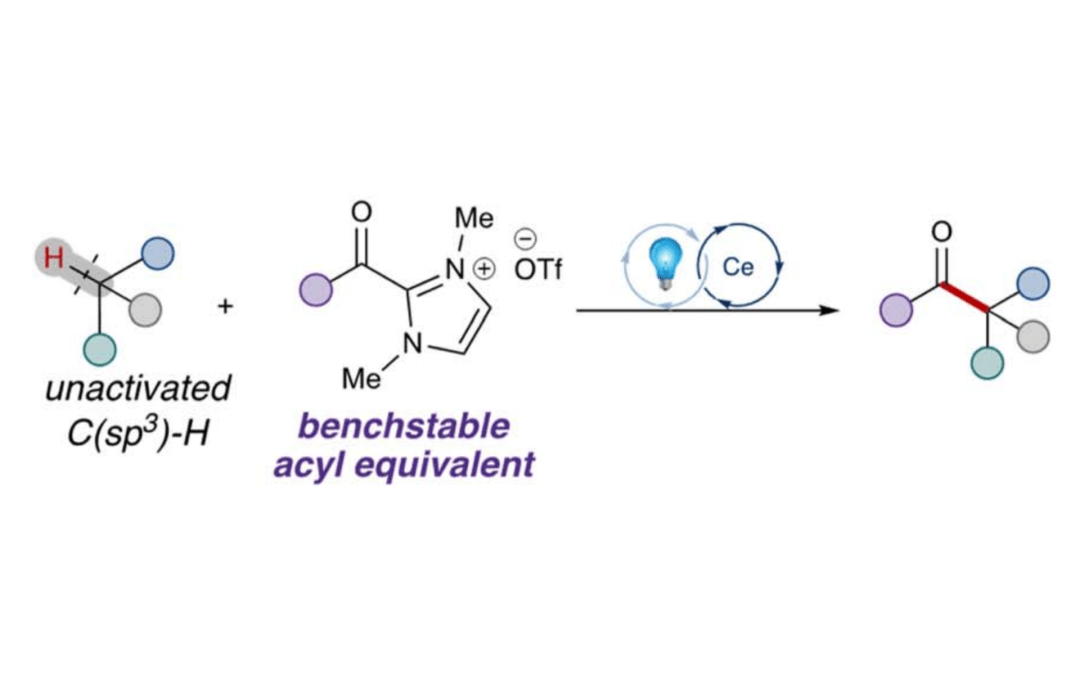
Photoinduced cerium-catalyzed C–H acylation of unactivated alkanes
Cao, J,; Zhu, J. L.; Scheidt, K. A.* Chem. Sci. 2023, 15, 154-159.
About Our Lab
We integrate chemical synthesis, catalysis and translational science to advance society and train the next generation of scientists.
One of our main goals in the Scheidt laboratory is expanding the field of chemistry through the discovery of catalytic reactions and creation of new molecules with unique properties and applications. Our new approaches for the synthesis of important molecules is an integral part of directing the power of organic synthesis towards investigating the interface between biology and chemistry.
About Karl Scheidt
Karl Scheidt is a Professor in the Department of Chemistry and Professor of Pharmacology in the Feinberg School of Medicine.
He is also the faculty director of ChemCore for the Robert H. Lurie Comprehensive Cancer Center. His research interests focus on developing efficient strategies to create new molecules with translational and clinical potential. As the founding Executive Director of the NewCures Accelerator, he is responsible for developing and leading strategies to build commercial value for therapeutic discoveries within Northwestern.
Our Group Members
Our group is a tight-knit community that thrives on the open exchange of ideas and discussion, fostering an environment of continual learning.
With diverse backgrounds, experiences, and training, the Scheidt Lab members are the core of a highly collaborative and engaging research environment, and we hold a strong emphasis on mentorship. Our team hails from all over the United States and abroad.