
Developing ICU Clinical Behavioral Atlas Using Ambient Intelligence and Computer Vision | NEJM AI


Elderly immunocompromised man with several years of intermittent dry cough but more green and productive over the last month. Carries diagnosis of asthma on symbicort and PE on eliquis. Afebrile, satting well on room air, but some bibasilar crackles.
Chest imaging showed scattered opacities, slightly reticular, more at the bases. He improved with CTX/azithro; after a week, grew a variety of NTM organisms from multiple samples, including M. abscessus (usually want to treat, more likely to progress and more virulent than MAC).
Group consensus was to send for susceptibilities at National Jewish, but not to treat given symptomatic improvement and repeat CT was much improved (near normal) without treatment for NTM.
Teaching point: Susceptibility showed azithromycin susceptible but high MIC of 32, concerning for something that would have inducible azithro resistance. Functional erm gene also a marker of difficulty to eradicate.
Ongoing discussions regarding treating (if so, with what? to what goal?) and immunosuppression adjustments…
Thanks, Dr. Olson!
Reposting this from last year as the LRP deadline comes up 11/16:
Today, first year fellow Jason Arnold shared an unusual case of pulmonary cryptococcosis in a HSCT recipient.
Here are a few high yield points from our discussion:


Thanks, Jason!
This week in ILD conference, Tim presented a case of fibrotic interstitial lung disease in a middle-aged woman who was born and raised in India and has lived in the US for several years. The CT showed diffuse fibrotic changes with air trapping but no particular apicobasilar gradient. CHP was suspected but HP panels negative and no clear exposure had been identified.
Dr. Parekh inquired whether the patient ever participated in preparation of chapati, which Tim & Anthony had not yet discussed with the patient. On follow up with the patient, she has made chapati every day of her adult life. Interestingly, she had not been involved in chapati baking in last 4-6 months because of hand arthritis. Interestingly, this time course correlated with a previously unexplained improvement in her symptoms.
I. What is the typical radiographic pattern associated with hypersensitivity pneumonitis?
Key point: hallmark of HP is air trapping – expiratory imaging as obtained in HRCT is essential for detection. Air trapping (hypoattenuation), alongside ground glass and normally perfused unaffected lung produces the pathognomonic “three density” or “head cheese” CT finding in HP
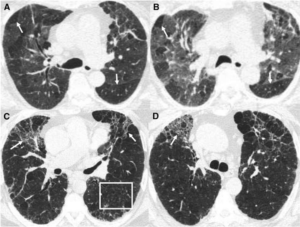
Figure: https://www.atsjournals.org/doi/10.1164/rccm.201608-1675PP
Non-fibrotic Hypersensitivity Pneumonitis: groundglass opacities in random axial and craniocaudal distribution; small (<5mm) centrilobular nodules, air trapping/mosaic attenuation.
Fibrotic Hypersensitivity Pneumonitis: similar distribution and pattern with interposed reticulation, traction bronchiectasis +/- honeycombing. Fibrosis tends to spare basilar lung zones, distinguishing from UIP pattern
HP diagnostic approach:

II. Besides exposure remediation, what is the standard pharmacologic treatment for CHP?
Key point: immunosuppression is the mainstay of pharmacologic treatment for hypersensitivity pneumonitis with evidence of active inflammation
III. What is “baker’s lung” and in whom should we suspect it? How does this differ from hypersensitivity pneumonitis from occupational exposure to flour?
Baker’s Lung: One of the most reported occupational lung diseases in western countries. It is characterized by respiratory symptoms such as airflow obstruction and bronchial hyper-responsiveness that has been shown to develop in work environments in which there is continued exposure to bakery flour dust.
Hypersensitivity pneumonitis: As a separate entity, Hypersensitivity pneumonitis has been reported in occupational exposure to bread flour, related either to precipitins against A fumigatus itself (a common culprit for HP in farmers lung), the flour mite Acarus siro, or weevil infestation.
Works Cited:
https://doi.org/10.1378/chest.13-1734 https://www.atsjournals.org/doi/full/10.1513/AnnalsATS.202009-1195CME https://www.atsjournals.org/doi/10.1164/rccm.201608-1675PP https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4278578/#R1 https://www.atsjournals.org/doi/full/10.1164/rccm.202005-2032ST https://www.sciencedirect.com/science/article/abs/pii/009167499290470M
Thank you Dr. Olson for a fantastic morning report!
A middle-aged man s/p transplant on sirolimus and tacrolimus presents with progressive dyspnea and fevers.
Chest CT with progressive ground glass opacities in bilateral upper lobes that has progressed now to extensive cystic/cavitary disease over the last few months, despite antibiotics.
BAL with positive histo antigen in serum, urine, BAL, pleural fluid, and on Karius testing! Improves with holding immunosuppression and itraconazole treatment.
This case may have been infectious, but thank you for the great teaching point on when to consider sirolimus toxicity:
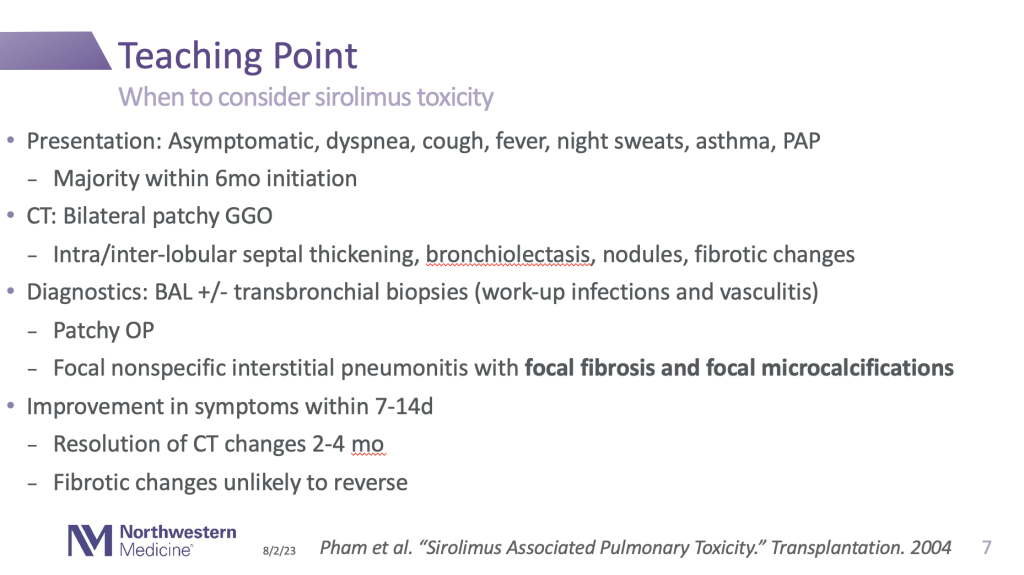
Thank you, Dr. Olson!

Today’s Pulmonary Report from Dr. Smith-Nuñez featured a case of chronic eosinophilic pneumonia which she treated on pulmonary consults.
First, she provided focused differential of pulmonary eosinophilia:

When thinking about eosinophilic pneumonia, what clues do we use to distinguish acute versus chronic?

Finally, a standard treatment regimen that was used in the care of our own patient:

Takeaways:
Pulmonary eosinophilia carries a broad differential including:
Chronic eosinophilic pneumonia (CEP) is characterized by:
Corticosteroids are mainstay of therapy, and relapses are common
Thanks, Ashley!

Tw: @dra_SmithNunez
Thanks to Dr. Szabo and Dr. Pickens for a fantastic grand rounds covering the new updated recommendations to severe CAP! [https://link.springer.com/article/10.1007/s00134-023-07033-8]
1) Suggest adding multiplex PCR to lower respiratory sample (very low evidence)
ResPOC trial [https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(17)30120-0/fulltext] – 720 patients presenting to ED with resp symptoms ran multiplex PCR and given information to clinical team -> slightly less abx (one dose vs 48hrs) and shorter LOS and good identification of viruses -> results 48-72hrs earlier than cultures, can quickly de-escalate or target resistance
Sputum biofires more often positive than BAL biofire (72% vs 49% and more likely polymicrobial, and also common discrepancy with cultures)
2) Consider HFNC instead of regular oxygen
RCT of standard oxygen facemask, HFNC, NIV [Frat NEJM 2015 https://pubmed.ncbi.nlm.nih.gov/25981908/] -> mortality benefit in those who got HFNC [though NIV might’ve been confounded because they targeted high tidal volumes]
3) Steroids? Based on their own meta-analysis, recommended if shock is concurrently present.
Didn’t include CAPE COD study, and driven by Meduri VA study [?confounded by gender]

Interesting thoughts on environmental ecology – examining the microbe in its true environment interacting with other bugs rather than taking it out and isolating it on a plate!
First, identify all microbes present [Human Microbiome Project – https://hmpdacc.org/]; there’s been a lot of interest recently!
The upper respiratory tract is different from the lower respiratory tract when you’re sick. Some features like bacterial burden or composition/diversity are associated with different outcomes!


Thanks, Dr. Szabo and Dr. Pickens!
Sharing my slides from Career Development Retreat: https://docs.google.com/presentation/d/1P9vcfwn8yLWfYL_6G4Dr8xrqsaN4iiW1wfgNGZqRg1A/edit?usp=sharing
Good luck; have fun!
Thanks to Jose for presenting this today! CAPE-COD (paper here)
Discussion points brought up included the variety of pathogens and heterogeneous groups and the syndrome of CAP, unclear exactly which subpopulation would be most benefitted (compared with COVID, where things were more clear and homogeneous), unusual population where a large number of patients had high CRP (based on a prior Spanish study that showed benefit in this population that took years to enroll a certain number of patients); steroids are a blunt instrument, choice of specific steroid (hydrocortisone vs dexametahsone). Of note – immediate meta-analysis incorporating this data – slight benefit?
Recent VA study showing no benefit (mostly men so maybe one explanation for the difference): https://link.springer.com/article/10.1007/s00134-022-06684-3
Of note – new guidelines for severe CAP including our very own Dr. Wunderink: https://link.springer.com/article/10.1007/s00134-023-07033-8
Future thoughts: designing trials better phenotyping to target specific pathways!