Case
A 70 year-old male is brought in by ambulance from his nursing home with 2 days of fever, shortness of breath and purulent sputum production. He is febrile, tachycardic, and tachypneic with an initial SpO2 of 86% on RA which improves to 92% with 5L NC. On exam he is alert and oriented but appears ill with crackles at his right lung base. A portable CXR shows a dense right-sided infiltrate. ABG does not show any evidence of hypercarbia
Questions
What oxygen delivery device would you use to help manage his hypoxemic respiratory failure? Is there a role for the use of a high-flow nasal cannula (HFNC)?
Physiology and recent trials
- Common O2 delivery devices
- In general, maximal flow rates of common O2 delivery device are limited by the ability to effectively heat and humidify gas at high flow rates
- Nasal cannula
- O2 delivery inefficient as O2 flowing through cannula mixes significantly with entrained room air (unable to obtain delivered FiO2 >40%)
- High flow rates poorly tolerated as cool dry air irritates the nares
- Face mask (Venturi mask)
- Can achieve higher flow rates (6-10L/min). Room air entrained through exhalation ports limits maximal FiO2 to 50%
- Non-rebreather mask
- Includes special valves which limit entrained air allowing delivery of FiO2 near 95%
- Maximal flow rates of 10-15L/min
- NIV (BiPAP)
- Good evidence to support the use of NIV in acute decompensated heart failure and exacerbations of obstructive lung disease
- Cumbersome to set up and interface often uncomfortable for patients
- Mixed data on its efficacy in acute hypoxemic respiratory failure (AHRF)
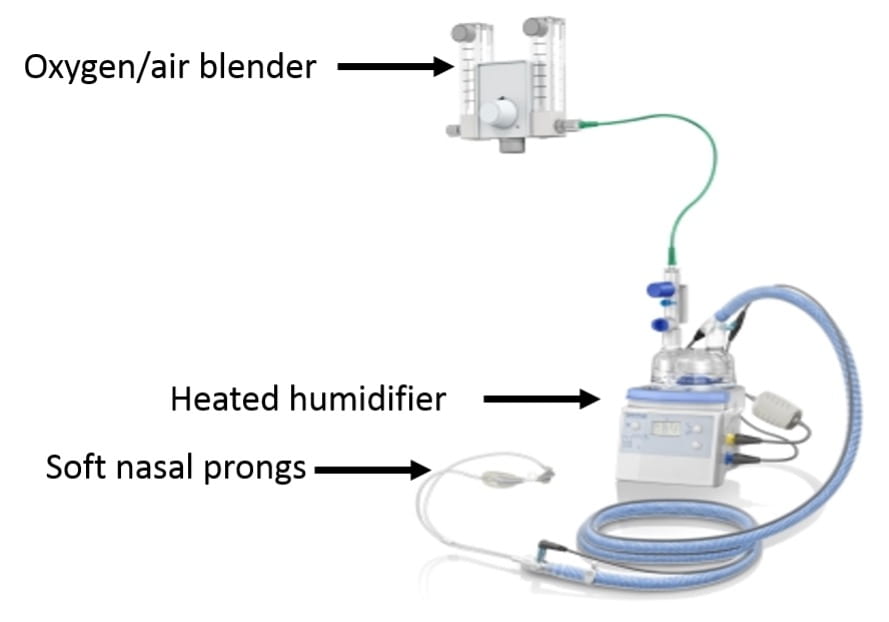
- HFNC
- Device (see picture above)
- Uses a special oxygen/air blender connected to a heated humidifier to saturate air with water and warm air to body temperature before delivery. This system allows for the delivery of very high flow rates (up to 60L/min)
- Connects to the nose with large soft prongs
- Potential benefits
- Ability to deliver heated and humidified gas at high flow rates
- Prevents drying of the airway and interference with mucocilliary clearance
- Enhances patient comfort
- May help moisten secretions making them easier to clear
- May lessen work of breathing by avoiding the bronchoconstricting effects of cold air and lessening the work needed to expectorate secretions
- Minimizing entrainment of room air
- Patients in respiratory failure often have high flow rates that exceed the flow of nasal cannulas and face-masks resulting in entrained room air which dilutes supplemental O2
- Flow rates in HFNC usually exceed patient-generated flows minimizing dilution of delivered oxygen
- Ability to deliver heated and humidified gas at high flow rates
- Potential benefits
- Device (see picture above)
- Improved ventilator efficiency
- HFNC can continually flush CO2 out of nasopharynx (lowering nasopharyngeal dead space) and allowing more of the minute ventilation to participate in gas exchange
- PEEP effect
- HFNC increases nasopharyngeal and esophageal pressure approximating levels seen with nasal CPAP
- HFNC may therefore provide a small amount of inspiratory assistance, help counter-balance auto-PEEP, and potentially improve oxygenation
- Breathing pattern
- Some evidence that HFNC helps increase TV and lowers RR
- Use
- Greatest benefit likely in patients with significant hypoxemia that would ordinarily be given standard high-flow oxygen therapy via a face mask
- Avoid in patients with high breathing workloads whose ventilatory failure is worsening (these patients require closely monitored NIV vs intubation)
- Usually provides more oxygen and gas flow than is necessary for patients with mild hypoxemia
- Initial settings
- Initial adjustments should be to flow rate as it is the flow rate that drives the physiologic benefit. Increase flow if RR fails to drop or if breathing remains labored with initial settings
- Starting flow rate is usually 35-40L/min
- Increasing flow rate should improve FiO2 as the amount of entrained air decreases
- There are two recent large randomized trials investigating the use of HFNC which are worth knowing
- High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure (FLORALI) – NEJM, 2015
- Methods
- Multicenter randomized trial in 23 ICUs throughout France and Belgium
- Inclusion (need to meet all 4)
- RR>25, P/F < 300 while on O2 flow of 10L/min for at least 15 minutes, PaCO2<45, no history of chronic respiratory failure (ie pts with AHRF without concurrent hypercapnia)
- Exclusions
- PaCO2>45, exacerbation of asthma or chronic respiratory failure, cardiogenic pulmonary edema, neutropenia, shock, GCS <13, contraindications to NIV, urgent need for intubation, DNR (important to exclude obstructive lung disease and decompensated CHF where there is a known benefit to NIV)
- Patients randomized to 3 groups
- Standard O2 therapy: nonrebreather mask with flow of 10L/min or more with flow adjusted to maintain SpO2 >92% until patient recovered or was intubated
- HFNC: flow rate >50L/min and FiO2 100% with FiO2 adjusted to maintain SpO2 >92%. HFNC applied for 48 hours. In the HFNC and standard oxygen group, a trial of NIV was allowed at the discretion of the MD
- NIV: pressure support adjusted to obtain a tidal volume of 7-10cc/kg PBW with initial PEEP between 2-10 cmH20. FiO2 and PEEP then adjusted to maintain SpO2 >92%. Minimum duration of NIV was 8 hours/day for at least 2 calendar days. NIV applied during sessions of at least 1 hour and could be resumed if RR was more than 25 and SpO2 <92%. Between NIV, patients received HFNC
- Results
- 2,500 patients admitted with HRF 525 eligible 313 randomized (13% of patients with HRF included in trial)
- Causes of respiratory failure
- CAP: 64%
- HAP: 12%
- Initial mean settings
- Standard O2: O2 flow rate of 14L/min
- HFNC: O2 flow rate of 48L/min, mean FiO2 80%
- NIV: 8/5, FiO2 70%, TV 9 cc/kg
- 25% in NIV group received therapy for <4hrs per day
- 40 patients in the HFNC and standard O2 received BiPAP as a rescue therapy
- Outcomes (statistically significant results in bold)
- Methods
- High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure (FLORALI) – NEJM, 2015
| HFNC | NIV | Standard O2 therapy | |
| intubation rate by 28 days (primary outcome) | 38% | 50% | 47% |
| intubation rate by 28 days in pts with P/F £200 | 35% | 58% | 53% |
| interval between enrollment and intubation | 27° | 27° | 15° |
| ventilator-free days at day 28 | 24 | 19 | 22 |
| ICU mortality | 11% | 25% | 19% |
| 90-day mortality | 13% | 31% | 22% |
- 90-day mortality
- Hazard ratio for death of 2.01 (1.01-3.99) when comparing standard O2 vs HFNC group and 2.5 (1.31-4.78) when comparing NIV vs HFNC
- Intensity of respiratory discomfort and dyspnea score significantly improved in the HFNC group 1 hour after enrollment
- Conclusions
- The use of HFNC vs standard O2 or NIV did not prevent the primary outcome of need for intubation at 28 days
- HFNC was associated with several important secondary outcomes
- Lower ICU mortality
- Lower 90-day mortality
- Less need for intubation in patients with a P/F <200
- The authors and editorial wonder whether the high tidal volumes achieved with NIV may have contributed to the worse secondary outcomes seen in the NIV group
- High-Flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery – JAMA, 2015
- Respiratory failure is common following cardiac surgery with NIV currently the treatment of choice. However, NIV is cumbersome, requires significant resources, and fails in 20% of patients. Authors wondered whether HFNC may be a better choice.
- Methods
- Multicenter randomized trial in 6 ICUs throughout France.
- Patients (any of the following)
- Failure of post-op SBT
- Successful SBT in patients with a BMI >30, LVEF <40%, or failure of prior extubations
- Successful SBT followed by failed extubation (P/F <300, RR >25 for at least 2hrs, use of accessory muscles)
- Exclusions included OSA, tracheostomy, DNI, delirium, nausea, vomiting, altered mental status, hemodynamic instability
- Patients randomized to the use of HFNC or NIV
- Results
- 3,217 eligible, 830 randomized (80% were s/p CABG)
- Primary outcome of treatment failure (defined as re-intubation, switch to other study treatment, or premature study discontinuation)
- 9% in BiPAP vs 21.0% in HFNC (no difference)
- No difference in time to treatment failure (1 day in each group)
- Roughly 14% required re-intubation in each group (no difference)
- No difference in outcome among patients with a P/F < 200 (in contrast to the NEJM study).
- Similar dyspnea and comfort score
- No difference in ICU or 28 day mortality
- Conclusion
- HFNC is not inferior to NIV in preventing treatment failure in patients with or at risk for respiratory failure following cardiothoracic surgery
My thoughts
- I find the results of the FLORALI study less convincing than the associated editorial suggests they are. The significant cross-over between groups (when not on NIV, patients in the NIV group received HFNC and 40 patients not on NIV were placed on NIV as a rescue therapy) and the variable duration of NIV therapy (25% of patients received therapy for <4 hours per day) make a clean comparison between the three O2 delivery methods difficult. If a patient is on NIV for <4 hours a day and the other 20 hours was managed with HFNC, should their outcome really be associated with NIV use?
- I also find it difficult to explain the improved 90-day mortality seen with HFNC. It is hard to picture an interaction between any mode of non-invasive O2 delivery and mortality that does not hinge on precluding the need for mechanical ventilation which was not seen in this trial. The authors of the trial and the editorial suggest that the high tidal volume achieved with NIV may have worsened lung injury thus leading to more time on the ventilator and increased mortality but it is hard to buy this conclusion if patients managed with NIV did not require intubation any more than patients in the other two groups nor did they have higher rates of refractory hypoxemia. A trend of P/F ratios in all three arms over time would have been helpful. Concerns have also been raised about the high rate of septic shock in the NIV arm (31%) vs the HFNC arm (18%)
- Important to note that 45% of patients required intubation by 28 days. This highlights the importance of closely monitoring patients with AHRF being managed with HFNC or NIV as roughly half will fail.
- After reviewing this topic, I find the use of HFNC most appealing in patients who would normally be managed with a higher-flow face mask (venturi or nonrebreather). The ability to deliver very high flows of heated/humidified air seems to offer real physiologic and comfort benefits over typical higher flow devices.
- We recently discussed the FLORALI study at a pulmonary conference. Faculty raised the important point that for older patients with pneumonia, difficulty with secretion clearance is often what leads to initiation of mechanical ventilation. In this group of patients, placing them on NIV may inhibit their ability to clear secretions and hasten respiratory failure. Specifically for this reason, Dr. Wunderink (MICU director) supported the idea of using HFNC over NIV as a first line therapy for older patients with pneumonia and resulting hypoxemic respiratory failure who do not require immediate mechanical ventilation.
- For those who manage patients with hypoxemic respiratory failure (either in the ER or in the ICU), if you never find yourself initiating therapy with HFNC, you are probably underusing a helpful tool.
Take-home points
- High flow nasal cannula devices deliver heated and humidified air at very high flow rates. Potential benefits include improved patient comfort, improved secretion clearance, a small PEEP effect, and washout of nasopharyngeal dead space
- The use of HFNC may improve long-term outcomes in patients with acute hypoxemic respiratory failure although further trials are needed to validate this finding
- HFNC is not inferior to NIV in preventing treatment failure following cardiothoracic surgery
Attached
- Helpful review of HFNC from CHEST, 2015
- FLORALI trial, NEJM, 2015
- Randomized trial of HFNC following cardiothoracic surgery, JAMA, 2015
















