Surface Chemistry and Cellular Control
Our group uses self-assembled monolayers (SAMs) modified with specific surface chemistries to design substrates that control cellular adhesion, shape, migration, and differentiation. We pair this with many technologies like microcontact printing to control cell geometry. As a postdoc in the Whitesides Group, Milan and his colleagues demonstrated that the spread area and geometry of cells intrinsically controlled their ultimate fate (i.e. growth or apoptosis). Early seminal work from the group demonstrated that the curvature of cell shape directly influenced stem cell differentiation pathways. More recent examples have evolved microcontact printing methodology on SAMs and shown that photodynamic surfaces can be directly controlled with light to control cellular shape. Furthermore, high-resolution nanoprinting has been explored for controlling focal adhesion location, pattern, and ultimately, cell shape, expression, and phenotype. Currently, work is being done to investigate if advanced machine learning algorithms can predict cell type and cancer metastatic potential based on the shape of the actin cytoskeleton.
Surface Chemistry
Patterning Techniques
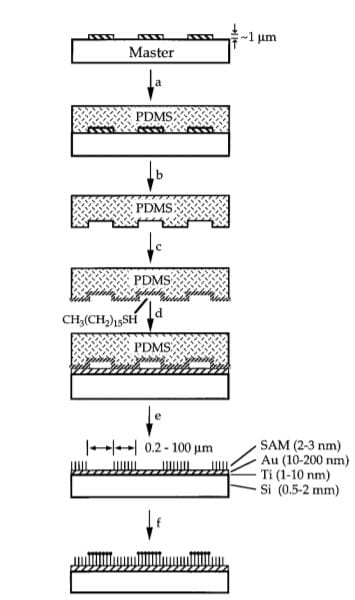
Utilizing the inherently adsorption-resistant characteristics of self-assembled monolayers (SAMs), our group has utilized and evolved the microcontact printing (uCP) method for restricting cell adhesion into specific shapes. Recently, our group has synthesized a photoactive peptide to increase resolution of cell patterning.
Engineering and Cell Biology
Application
With successful cell patterning, our platform allows us to strudy fundamental cell biological phenomena such as cell adhesion formation dynamics, stem cell differentiation, and gap junction formation. Furthermore, we can use high-resolution confocal microscopy image acquitision to augment analysis.
Patterning Techniques
As a postdoc in the Whitesides Group at Harvard, Milan and colleagues demonstrated that the physiochemical technique of microcontact printing (right) could be combined with the inertness of self-assembled monolayers (SAMs) to create superiorly defined regions of adhesion to study cell biology.
Fundamentally, they found that specific shape and decreasing cell area led to increased cell apoptosis for endothelial cells. This has led to our group working to evolve the intial uCP method in order to increase the resolution, consistency, and throughput of cell patterning.
Initially, work on the group focused on electrically active substrates that could turn adhesion on/off by releasing blocking/adhesive ligands, often peptides. This allowed for selective patterning of multiple cell populations.
Most recently, work in our group has led to using a photoactive peptide that, when exposed to high energy wavelength (405 nm) light from a confocal microscope, allows for improved and more selective adhesive regional resolution against an intert backdrop (bottom). Here, photoactivating a region between two a priori patterned cells allowed for gap junction dynamic formation analysis.

Key Patterning Papers:
B. T. Houseman & M. Mrksich. An Efficient Solid-Phase Synthesis of Peptide-Substituted Alkanethiols for the Preparation of Substrates that Support Adhesion of Cells. J. Org. Chem. 63, 7552-7555 (1998)
Y.-Y. Luk, M. Kato and M. Mrksich. Self-Assembled Monolayers of Alkanethiolates Presenting Mannitol Groups are Inert to Protein Adsorption and Cell Attachment. Langmuir, 16, 9604-9608 (2000)
B. T. Houseman & M. Mrksich. The Microenvironment of Immobilized Arg-Gly-Asp Peptides is an Important Determinant of Cell Adhesion. Biomaterials, 22, 943-955 (2001)
M. N. Yousaf, B. T. Houseman and M. Mrksich. Turning on Cell Migration with Electroactive Substrates. Angew. Chem. Int. Ed. 40, 1093-1096 (2001)
M. N. Yousaf, B. T. Houseman and M. Mrksich. Using Electroactive Substrates to Pattern the Attachment of Two Different Cell Populations. , Proc. Natl. Acad. Sci. USA. 98, 5992-5996 (2001)
W.S. Yeo, M.N. Yousaf, M. Mrksich. Dynamic Interfaces Between Cells and Surfaces: Electroactive Substrates that Sequentially Release and Attach Cells. J. Am. Chem. Soc. 125(49), 14994-14995 (2003).
W. L. Murphy, K.O. Mercurius, S. Koide, M. Mrksich. Substrates for Cell Adhesion Prepared via Active Site-directed Immobilization of a Protein Domain. Langmuir. 20(4), 1026-1030 (2004)
M. Kato & M. Mrksich. Rewiring Cell Adhesion. J. Am. Chem. Soc. 126(21), (2004)
M. Kato & M. Mrksich. Using Model Substrates To Study the Dependence of Focal Adhesion Formation on the Affinity of Integrin-Ligand Complexes. Biochemistry, 43(10), 2699 (2004)
R.T. Petty, H-W. Li, J.H. Maduram, R. Ismagilov and M. Mrksich. Attachment of Cells to Islands Presenting Gradients of Adhesion Ligands. J. Am. Chem. Soc. 129(29), 8966-8967 (2007)
S. Raghavan, R.A. Desai, Y. Kwon, M. Mrksich, C. S. Chen. Micropatterned Dynamically Adhesive Substrates for Cell Migration. Langmuir. 26, 17733-17738 (2010)
Bugga, P. & Mrksich M. Sequential Photoactivation of Self-Assembled Monolayers to Direct Cell Adhesion and Migration. Langmuir, 35, 17, 5937-5943 (2019)
Application
Once the microcontact printing on SAM technique was perfected and evolved, it allowed our group to study many fundamental scientific phenomena rooted in cell biology.
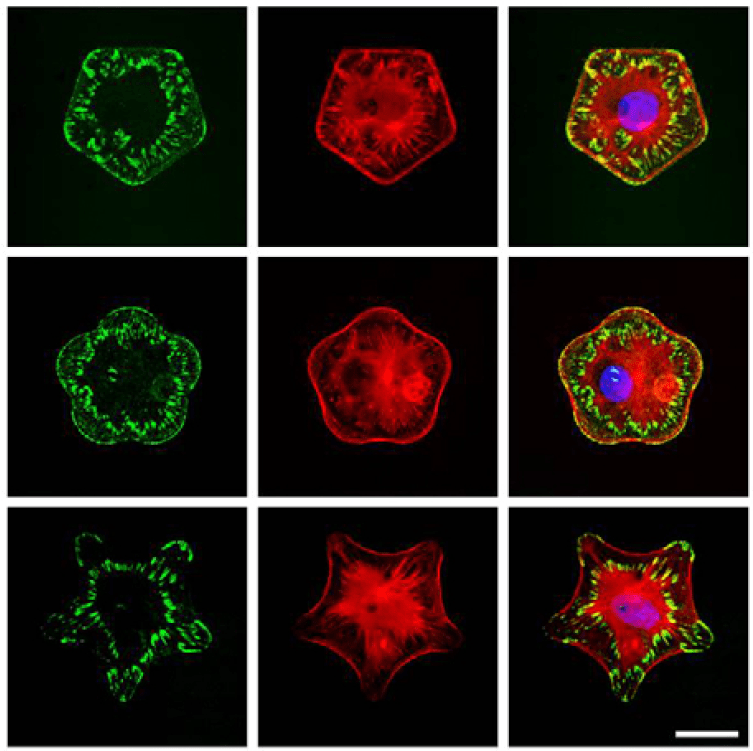
Our group’s landmark paper in this respect came in 2010 where we demonstrated how geometric cues influenced stem cell differentiation. In essence, not only was the cell shape and aspect ratio investigated, but keeping area constant, the curvature of the cell shape had a profound influence on the differnentiation pathway human mesenchymal stem cells undertook. The shape and curvature that allowed for pronounced and aligned actin cytoskeleton led cells to adopt a more osteogenic profile while diffuse cytoskeletons led cells to adopt a more adipogenic profile.
Recent collaborative work in the group has focused on understanding how anisotropic adhesion formation leads to contractile actin cytoskeletons and cell behavior.
Application

Figure 2. Anisotropic adhesion formation via microcontact printing leads to differential cell phenotypes and behaviors.
Key Application Papers:
James, J., Goluch, E.D., Hu, H., Liu, C., and Mrksich, M.. Subcellular Curvature at the Perimeter of Micropatterned Cells Influences Lamellipodial Distribution and Cell Polarity. Cell. Motil. Cyctoskel., 65, 841-852 (2008)
Kilian, K.A., Bugarija, B., Lahn, B., Mrksich, M.. Geometric Cues for Directing the Differentiation of Mesenchymal Stem Cells. , Proc. Natl. Acad. Sci. 107(11), 4872-4877 (2010).
Kilian, K.A., and Mrksich, M. Directing Stem Cell Fate by Controlling the Affinity and Density of Ligand-Receptor Interactions at the Biomaterials Interface. Ang. Chem. Int. Ed. 51, 4891-4895 (2012)
Shabbir, S.H., Cleland, M.M., Goldman, R.D., and Mrksich, M. Geometric Control of Cytoskeletal Elements: Impact on Vimentin Intermediate Filaments. Biomaterials, 35, 1359-1366 (2014)
Cabezas, M.D., Eichelsdoefer, D.J., Brown, K.A., Mrksich, M. and Mirkin, C.A. Combinatorial Screening of Mesenchymal Stem Cell Adhesion and Differentiation Using Polymer Pen Lithography. Methods in Cell Biology. 119, 261-276. (2014)
Muni, T., Mrksich, M., and George, A. Self-Assembled Monolayer Facilitates Epithelial-Mesenchymal Interactions Mimicking Odontogenesis. Connective Tissue Res. 55, 26-33. (2014)
Sobers, C.J., Wood, S.E., and Mrksich, M. A Gene Expression-Based Comparison of Cell Adhesion to Extracellular Matrix and RGD-Terminated Monolayers. Biomaterials, 52, 385-394 (2015)
Burbulla, L.F., Beaumont, K.G., Mrksich, M. and Krainc, D. Micropatterning Facilitates the Long-Term Growth and Analysis of iPSC-Derived Individual Human Neurons and Neuronal Networks. Advanced Healthcare Materials. 5, 1894-1903 (2016)
Cabezas, M.D., Meckles, B., Mirkin, C.A. and Mrksich, M.. Subcellular Control Over Focal Adhesion Anisotropy, Independent of Cell Morphology, Dictates Stem Cell Fate. ACS Nano, 13, 11144-11152 (2019)
