 | Analytical HPLC This Agilent 1100 series high pressure liquid chromatography system is equipped for high sensitivity sample analysis. Features: Diode-array absorbance detector from 190 to 950nm. Fluorescence detector. Peltier-thermostated auto-sampler (4-40 degrees C). Programmable automatic injector for 20- 900μl sample. Vacuum degasser. Peltier-thermostated column compartment (4-80 degrees C). Quaternary pump system using a high-speed proportioning valve. Note: The Keck Facility does not supply HPLC columns. Location: Cook Hall, 4106. NU Core ID: HPLC Agilent Technologies |
 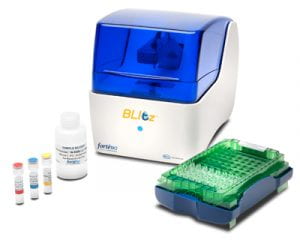 | BLItz Bio-layer Interferometer The BLItz is a micro volume instrument for characterizing the kinetics of macromolecular interactions using bio-layer interferometry with low cost disposable sensor probes. Overview BLItz™ uses ForteBio’s Dip and Read™ label-free assays. These direct binding assays take place on a disposable biosensor made from a biocompatible matrix that is uniform, non-denaturing and minimizes non-specific binding. Only molecules that bind directly to the biosensor surface are detected, providing exceptional specificity for individual applications, even in crude media.  The BLI (bio-layer interferometry) technology used by BLItz provides real-time data on protein interactions. BLItz emits white light down the biosensor, and then collects any light reflected back. Reflected wavelengths are affected by the thickness of the coating on the optical layer. Some wavelengths show constructive interference (blue), others show destructive interference (red).  This interference is captured by a spectrometer as a unique spectral signature and is reported in relative intensity units (nm). Any change in the number of molecules bound to the biosensor causes a shift in the interference pattern that is measured in real time. This wavelength shift is a direct measure of the change in optical thickness (nm) of the biological layer. Biosensor Types For a list of all available biosensor types, please see here. Note that our facility has select sensor types (please check with us to confirm the specifics) available for purchase to users. An instrument is located on each campus. Location: Cook Hall, Rm 4106, Evanston Campus Location: Tarry Building, Rm 7-710, Chicago Campus - Not Available NU Core ID: Bio-Layer Interferometer (BLITZ) Evanston |
 | Differential Scanning Calorimeter The MicroCal (GE Healthcare) differential scanning calorimeter directly measures the enthalpy of a solution as a function of temperature. Applications of the DSC technique: Protein stability and folding. Protein engineering. Characterization of membranes, lipids, nucleic acids and micellar systems. Assessment of the effects of structural change and ligand binding on a molecule’s stability. Instrument Characteristics: Active cell volume ~ 0.5 ml. Non-reactive Tantalum 61™ cells for excellent chemical resistance. Fixed-in-place cells for reproducible ultrasensitive performance with low maintenance. Operating temperature range of –10ºC to +130ºC. Peltier element for precise temperature control. User selectable temperature scan rates (0ºC to 90ºC per hour upscans) and range for application versatility. Location: Cook Hall, 4106 NU Core ID: DS Calorim MicroCal | VP-DSC |
 | Isothermal Titration Calorimeter The MicroCal (GE Healthcare) ITC200 isothermal titration calorimeter is a powerful tool for label-free analysis of macromolecular interactions in solution. By measuring the amount of heat released or taken up during each reaction in a titration experiment, the equilibrium binding affinity and the stoichiometry are derived; in addition, ITC is the only technique able to directly measure the thermodynamic parameters of binding (enthalpy, entropy and heat capacity changes) – providing therefore invaluable insights into the thermodynamic forces governing biomolecular interactions. The technique is suitable for analyzing protein-protein, protein-DNA/RNA interactions as well as interactions between biopolymers or synthetic macromolecules and small ligands. -Affinity constants from 100 to ~109 M-1 can be measured by ITC. -The sample cell volume is approximately 200 μl; minimum sample volume is 290 μl. -The syringe volume is 40μl. -The operating temperature range is 2°C to 80°C. Location: Cook Hall, 4118 NU Core ID: ITC200 MicroCal | ITC200 |
 | Affinity ITC The new Affinity ITC ( TA Instruments) is a powerful tool for measuring a wide variety of molecular interactions. It brings advanced engineering to all critical aspects of the measurement ensuring the highest quality ITC data. Features -AccuShot™ delivers the titrant to the right location for the best mixing -FlexSpin™ provides innovative slow speed stirring, efficient mixing and highest sensitivity -Fully automated, user-selectable system cleaning routines eliminate run-to-run contamination -Intelligent Hardware Positioning for precise reliable injections -Solid-state active heating and cooling for true isothermal temperature control -Low volume cells (190 µL) -Powerful ITCRun and NanoAnalyze for the most comprehensive suite of tools for method optimization, model fitting, batch analysis, graphing and data export. NU Core ID : Affinity ITC Contact Keck Biophysics for more information TA Affinity ITC |
 | NanoDLS pUNK The pUNK, or NanoDrop DLS, from Unchained Labs offers a fast and easy method to measure protein size, aggregation, and polydispersity. The smallest, fastest and easiest to use protein sizing system. Only 5 µL of sample required to determine: Percent aggregation. Polydispersity. Location: Cook Hall, 4118 NU Core ID: Punk More Information |
 | SEC-MALS-QELS The SEC-MALS-QELS system is composed of a size exclusion chromatography system (SEC) followed by a Wyatt DAWN HELEOS II multi-angle static light scattering detector, a Wyatt QELS dynamic light scattering detector, and a Wyatt T-rEx differential refractive index detector. System components: Wyatt DAWN HELEOS II – multi-angle (static) light scattering (MALS) detector. The molar mass and root mean square radius of the solute are determined by measuring the intensity of the scattered light – provided that the refractive index increment (dn/dc) of the solute/solvent system is known. Wyatt Optilab T-rEx – refractometer for measuring the absolute refractive index and its increment Wyatt QELS – quasi-elastic (dynamic) light scattering (QELS) detector. The diffusion coefficient/hydrodynamic radius can be determined from the temporal fluctuations in the intensity of scattered light HPLC – with size-exclusion columns (SEC), DAD absorbance, and fluorescence detectors In this set-up the sample components are separated according to size and characterized individually by static and dynamic light scattering, absorbance and fluorescence. Typical applications: For proteins and nucleic acids: measurements of molecular weights and radii, oligomerization, heterogeneity and solubility studies, characterization of macromolecular complexes in terms of size, overall shape and composition/stoichiomentry. For synthetic polymers: measurements of molar mass & mass moments, polydispersity, conformation/branching. For nanoparticles: solubility and size distribution measurements and composition /stoichiometry analysis for functionalized nanoparticles. More Applications Location: Cook Hall, 4106 NU Core ID: MALS QELS |
 | Octet K2 Bio-Layer Interferometer The Octet K2 System characterizes protein-protein and protein-small molecule binding interactions for early research and discovery. The multipurpose system also provides quantitative information by measuring active protein concentrations — even in complex mixtures like cell culture supernatants and lysates. Features: Sensitivity for Small Molecule Analysis The Octet K2 System enables the measurement of interactions between small molecules down to 150 Da in size, and their larger binding partners to determine accurate kinetics rates. Flexible Second Channel for Use as a Reference or More Samples Unique 2-channel design have dedicated spectrometers for simultaneous referencing or added capacity. Simple Setup for Protein and Antibody Characterization Template driven interface allows new users to get started quickly for protein and antibody characterization. Location: Cook Hall, 4118 NU Core ID: BLItz Bio-Layer Interferometer Octet |
  | Surface Plasmon Resonance The Reichert 4 SPR system is a four channel surface plasmon resonance spectrometer for label-free measurement of macromolecular association and dissociation kinetics. SPR is suitable for a wide range of interactants including proteins, nucleic acids, lipids, carbohydrates, small drugs and synthetic polymers and nanoparticles. The Reichert4SPR 4-Channel Surface Plasmon Resonance system allows users to accelerate and enhance the drug discovery process in the following ways: -Easy set-up and streamlined workflow. -Utmost sensitivity and maximum uptime. -Robust fluid handling system to run demanding assays. Features: 4 channel channel acquisition. Autosampler holding up to 96 HPLC vials or two 96 or 384 well plates. Controllable injection volume to conserve precious analytes. Optional sampling cooling for extended assays. Interaction temperature controllable between 10°C and 70°C. Detection of small molecule analytes down to ~100 daltons. Accessible range of binding affinities from 1 mM to 1 pM. Immobilization Techniques: Amine coupling to carboxylated planar monolayer or branched dextran sensor chips. Thiol coupling using a native or introduced cysteine. NeutrAvidin, streptavidin, or avidin capture of biotinylated molecules. Nickel NTA capture of histidine-tagged molecules. Protein A capture of antibodies. Lipid monolayer formation on a hydrophobic surface. Reichert provides an assortment of sensor chips to accommodate different immobilizations. Please see here for a complete list of available sensor chips. Location: Cook Hall, 4106 NU Core ID: SPR More Information |














