A highly technology-oriented regenerative medicine lab employing in vivo and in vitro models of nerve degeneration and regeneration.
What is Regenerative Medicine?
The goal of regenerative medicine is to boost innate repair processes and/or to replace tissues to improve function. Incorporation of regenerative principles into practice arguably has more relevance physical medicine and rehabilitation (PM&R) than any other medical specialty.
About Our Lab
As a PM&R-physician and scientist with sub-specialty certifications in neuromuscular and electrodiagnostic medicine, the focus of Franz’s clinical and research agendas are highly integrated. He studies as well as takes care of patients with traumatic neurological injuries, neuropathy, diaphragm muscle weakness and amyotrophic lateral sclerosis (ALS). The Franz lab uses a combination of cellular, molecular, bioengineering and electrophysiological techniques and employs small animal (in vivo) and human stem cell-based (in vitro) models of nerve degeneration and regeneration.
Areas of Focus
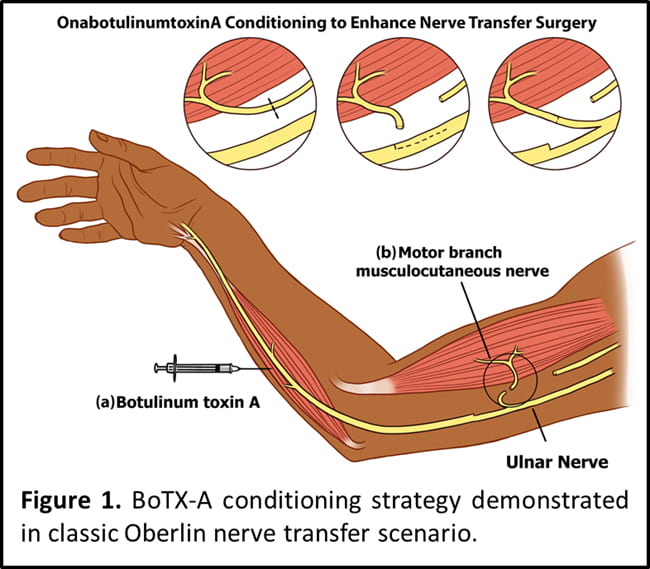 Developing activity-based treatments to augment peripheral axon regeneration.
Developing activity-based treatments to augment peripheral axon regeneration.
For example, pre-conditioning motor nerves with intramuscular botulinum toxin injection (“donor” site) prior to motor nerve transfer surgery (Figure 1; see also Franz et al. NNR 2018).
Determining the impact of human genetic variants/mutations on neurorehabilitation outcomes after neurotrauma
Figure 2. Understanding the relationship between amyotrophic lateral sclerosis (ALS) and neurotrauma with patient-specific induced pluripotent stem cells (iPSCs)-based neurons. Induced pluripotent stem cell-derived neurons could be used in combination with an in vitro model of neurotrauma to understand gene-trauma interaction in ALS as follows. A: stretchable 96-well plates are fabricated by bonding a layer of flexible, transparent 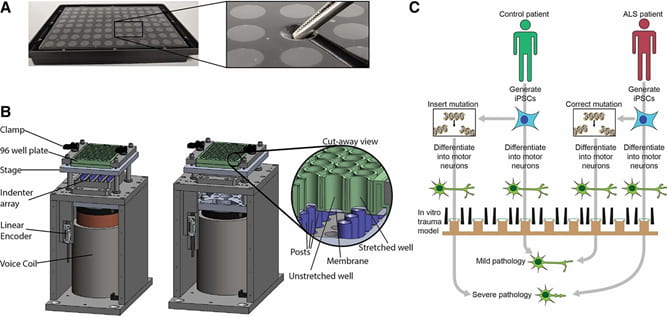 silicone to bottomless 96-well plates. The inset shows tweezers gently depressing on of the well bottoms to illustrate the flexibility of the growth substrate. B: a custom-built device is used to apply a rapid, repeatable, equibiaxial stretch to the bottom of the wells. Stretch is produced by pressing the plate rapidly down against an array of lubricated, Teflon-coated, cylindrical indenters. The cut-away view shows an example of a stretched well alongside a well that is not stretched because the corresponding post is not present. C: schematic of an experimental design to study gene-trauma interactions in ALS using this human in vitro model. A mutation associated with ALS can be engineered into an iPSC line derived from a healthy control individual or alternatively can be corrected in an iPSC line derived from a patient with a known disease-causing genetic variant. Both lines are then subjected to identical trauma in vitro, and subsequent pathology is quantified and compared with test the hypothesis that the ALS-associated mutation amplifies the pathology of trauma.
silicone to bottomless 96-well plates. The inset shows tweezers gently depressing on of the well bottoms to illustrate the flexibility of the growth substrate. B: a custom-built device is used to apply a rapid, repeatable, equibiaxial stretch to the bottom of the wells. Stretch is produced by pressing the plate rapidly down against an array of lubricated, Teflon-coated, cylindrical indenters. The cut-away view shows an example of a stretched well alongside a well that is not stretched because the corresponding post is not present. C: schematic of an experimental design to study gene-trauma interactions in ALS using this human in vitro model. A mutation associated with ALS can be engineered into an iPSC line derived from a healthy control individual or alternatively can be corrected in an iPSC line derived from a patient with a known disease-causing genetic variant. Both lines are then subjected to identical trauma in vitro, and subsequent pathology is quantified and compared with test the hypothesis that the ALS-associated mutation amplifies the pathology of trauma.