- Charged polymeric structures in biology and polymer physics.
- Stimuli-responsive polymeric systems.
- Atomistic (Gromacs) and course grained MD simulations (Lammps).
- Hydrodynamic effects in biological systems
- (Bio)polymer self-assembly
- Renormalization-Group theory, Ising models and hierarchical lattice
Kinetics of DNA-protein interactions
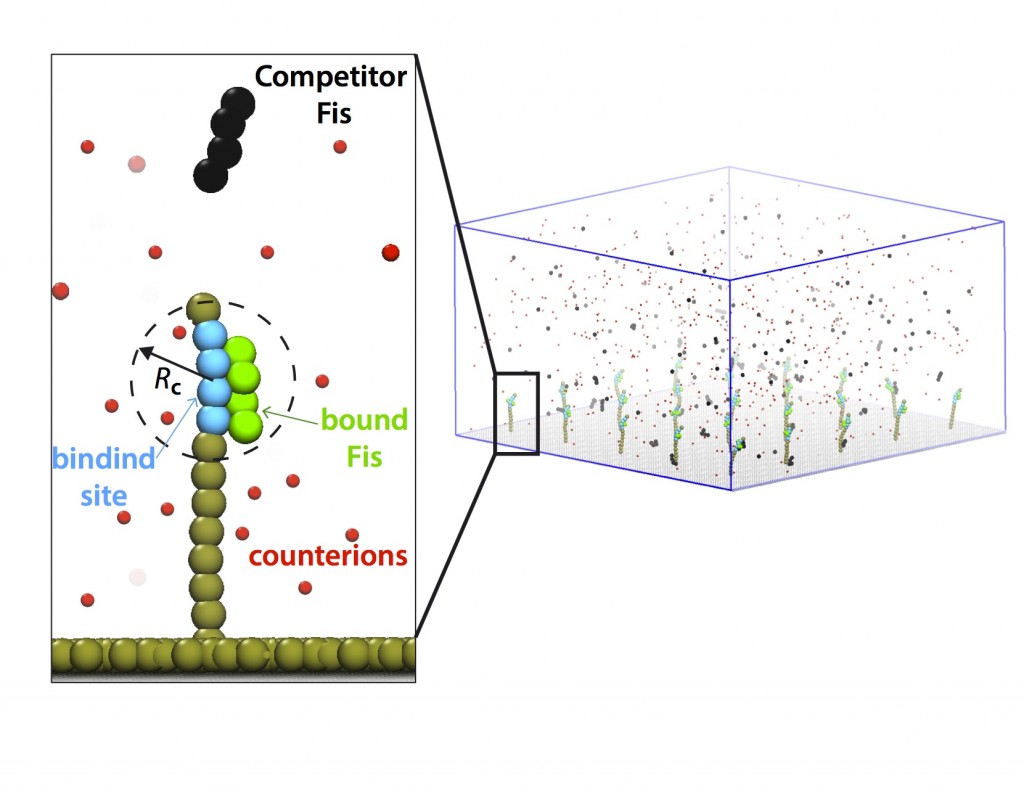 Various nuclear proteins such as Fis and NHP6A interact with dsDNA. Their binding rates and lifetimes contain important information on fundamental working principles and malfunctions in living cells. By collaborating with Marko lab at Northwestern University, I have worked on the results of the single-binding-site experiments and proposed an analytical binding model based on the modulation of electrostatic binding energies (Kamar, et al, 2016). Our results will shed light on rate-limiting factors in DNA-protein kinetic in a general scope and can help optimize certain bio-technological applications (e.g., bio-sensors) due to the prevalence of this phenomenon.
Various nuclear proteins such as Fis and NHP6A interact with dsDNA. Their binding rates and lifetimes contain important information on fundamental working principles and malfunctions in living cells. By collaborating with Marko lab at Northwestern University, I have worked on the results of the single-binding-site experiments and proposed an analytical binding model based on the modulation of electrostatic binding energies (Kamar, et al, 2016). Our results will shed light on rate-limiting factors in DNA-protein kinetic in a general scope and can help optimize certain bio-technological applications (e.g., bio-sensors) due to the prevalence of this phenomenon.
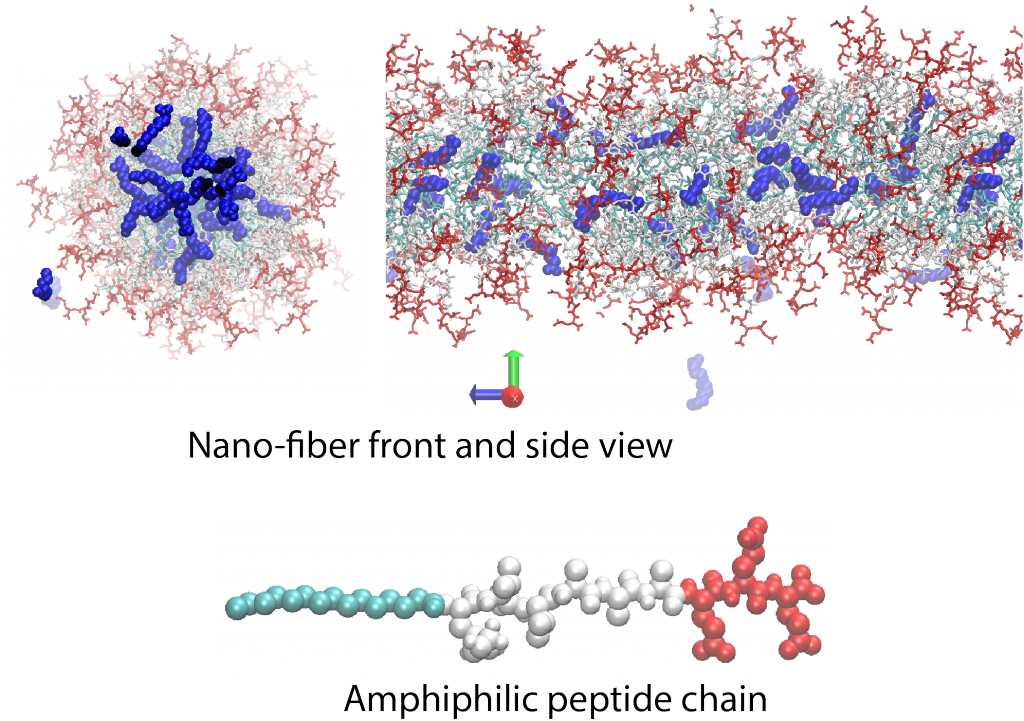
Seld-assembled peptide amphiphilious nanofibers
An amphiphilious peptide molecule is composed of hydrophilic (red) and hydrophobic (cyan and white) building blocks. These molecules can self-assemble and form 2D and 3D structures, such as micron-size nano-fibers, micelles or bilayers depending on solution pH, hydrogen bonding capacity of peptides (sequence) or salt concentration. Non-covalent nature of these structures as well as their amino-acid building blocks highlight them for pharmaceutical applications where bio-degradebility is paramount. We, in collaboration with Samuel Stupp Group, investigate the fatty acid (blue molecules in the snapshots shown above) encapsulations capacity of these nano-structures for pharmaceutical applications using atomistic molecular dynamic simulations and analytical theories.
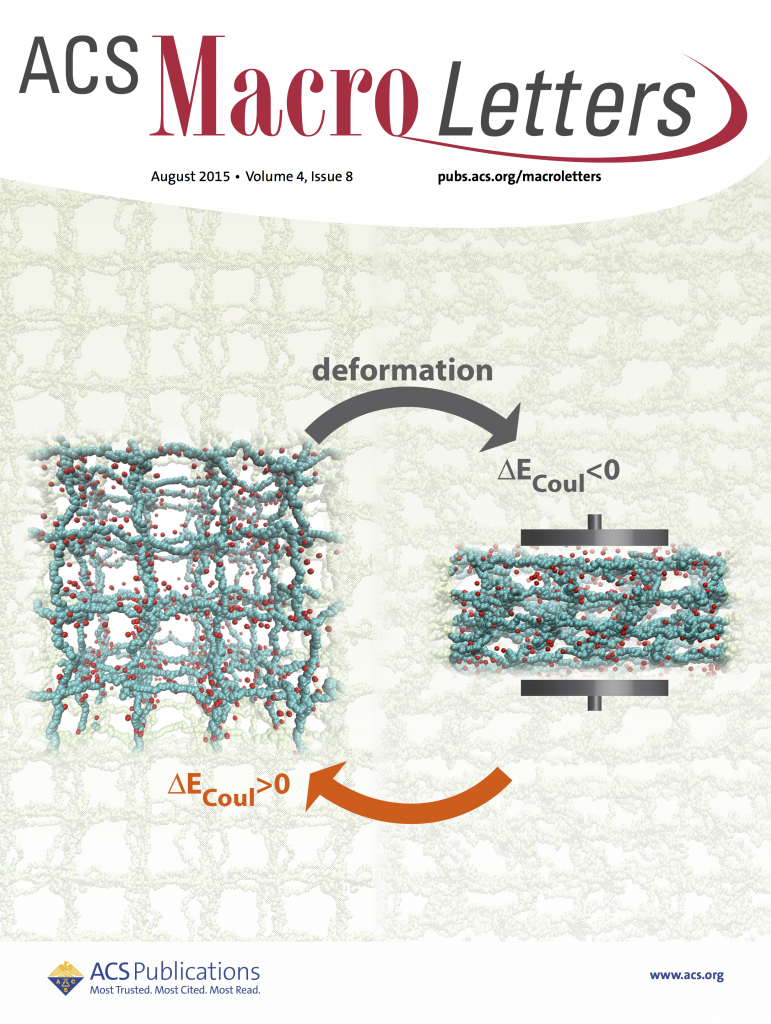 Polyelectrolyte hydrogels; energy conversion (with Monica Olvera de la Cruz)
Polyelectrolyte hydrogels; energy conversion (with Monica Olvera de la Cruz)
Hydrogels are highly swollen networks of charged polymers. Apart, from being the main constituent of contact lenses, pill capsules, etc., they have also proven themselves pretty effective in other applications, such as mechano-electric energy converters, energy storage and even as bio-mimetic structures. The balance between these inherent electrostatic and elastic properties of hydrogels allows them to adjust their internal electrostatic interactions reversibly upon application of external electromagnetic fields or mechanical deformations. Our MD simulations with explicit counterions suggest that if a hydrogel undergoes strain-control deformations, an amount of mechanical energy applied is converted into electrostatic energy rather than elastic and excluded volume energies. The energetic change is due to the decreasing translational entropy of counterion gas trapped inside the gel in combination with polymeric features of PE gels. This scenario is only possible if ionized counterions cannot vacate the gel, which is ensured by the electroneutrality condition inside the gel.
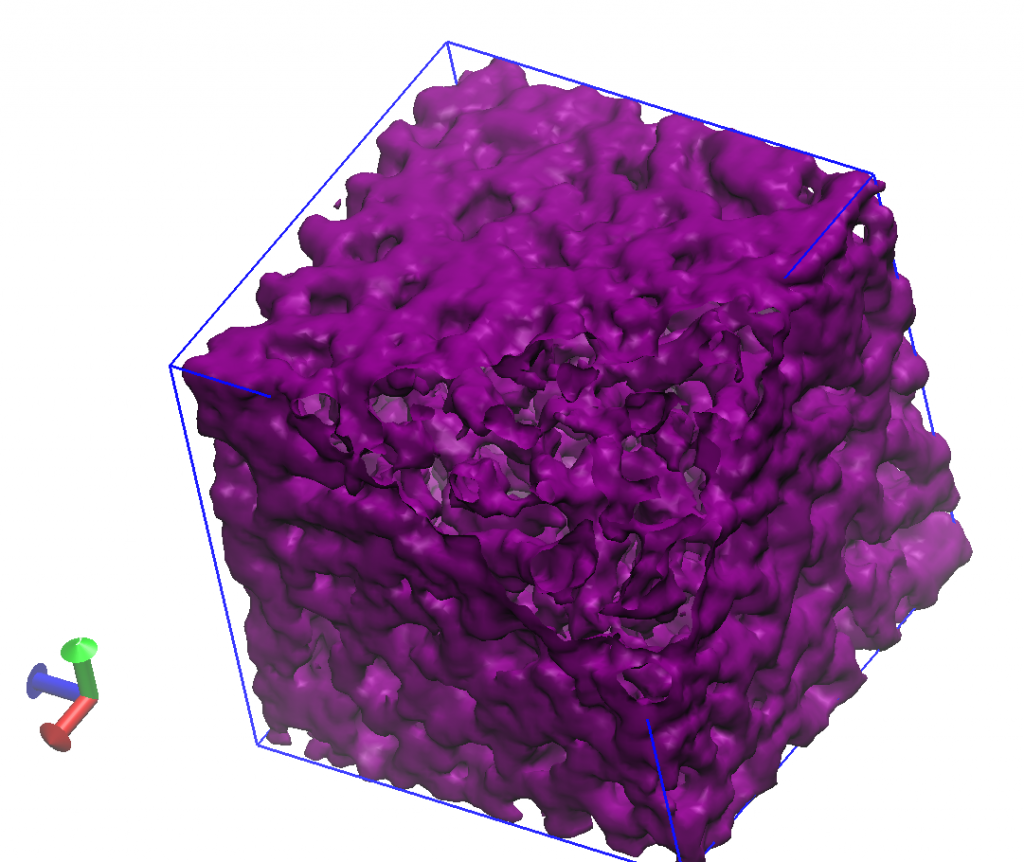
Amphiphilic Ionic Liquids for Advanced Electrolyte Applications (with Monica Olvera de la Cruz)
Ionic liquids (IL) are molten salts or equivalently pure ionic solvents. They exhibit high ionic conductivity at ambient temperatures. Combined with their low-volatility, electrochemical stability, inflammability, ILs are ideal candidates for wide range of electrochemical applications from energy-storage devices (battery electrolytes), display technologies to fuel cells, just to name a few. Our Goal in our research group is to investigate various structures and interaction parameters in Amphiphilic Ionic Liquids (AILs) using Molecular Dynamics (MD) simulations and various analytical tools. Provided a correct chemical structure, individual IL molecules can arrange to form liquid crystalline phases and ionic channels (shown above) through which charge carriers can move in response to potential differences . These systems can inspire the design of new generation ILs with high ionic conductivities and mechanical strengths.
Polymer brushes (with Michael Rubinstein)
Polymer-brush systems have been attracting scientists for last two decades extensively. On the other hand polyelectrolytes, which are polymer chains of charged monomers, have unique properties and are quite abundant in biological systems; DNA and most proteins can be counted among many. Experiments and simulations have shown that the friction coefficient between two polyelectrolyte brushes is less then 10^-4, which make them an excellent candidate for effective build-in lubricants. We would like to use coarse-grained computer models to understand and invoke further effects of other factors such as salt, velocity, etc. on polyelectolyte brush friction. Our computer models can also be used to check various scaling arguments quantitatively as well as quantitatively. Furthermore, since polyelectolyte brushes carpet the surface of the cilia, our simulations can shed light on cilia-mucus systems as well.
Friction in biological systems (with Roland R. Netz and Dominik Horinek)
In many daily-life engineering applications, friction is a drawback to be minimized for efficiency. Conversely, in biological systems involving self assembled building blocks held together by non-covalent interactions, friction is necessary to maintain the biological machinery. For instance, it is the friction that determines the ligand-receptor interactions or the folding times of proteins together with their free energy landscape. Similarly, for iso-energetic processes, for which the free energy barrier is either absent or low, such as a kinesin motor sliding on a microtubulin or a macromolecule diffusing on a surface, the friction manifests itself in the corresponding diffusion coefficient. To characterize the friction in such biological processes is more challenging compared to macro-solid systems, such as two blocks sliding relative to each other, where a single friction constant quantifies the friction. Read more here.
However, for biological soft systems, friction depends on the conformations of the molecules since these conformational changes effect the intra and inter-molecular interactions. For example, in a protein folding process, where the folding is preceded by an initial collapse of the amino acid chain, the number of native contacts increases and the water contact decreases as the folded state is approached. Not surprisingly, the confinement level of monomers by other monomers increases towards the folded state, and so does the friction. Read more here.

Air-Surface layers: Two main components of clearance mechanisms in the human respiratory system are viscoelastic mucus and cilia. The mucus layer driven by antiplectic beats of cilia not only cleans the respiratory tract system, bu also protects the epithelial layer from external factors such as viruses. Our aim is to combine concepts of polymer physics and hydrodynamics to build an understanding of the mucus-cilia machinery. Such a minimal model, which can reproduce in vivo as well as in vitro experimental results, can draw boundaries between healthy and diseased systems and quantify the mucus-cilia interactions in more detail.
See also our group page at http://tech.northwestern.edu/index.html