Covalent Organic Frameworks: Designed Porous Polymers
Covalent organic frameworks (COFs) are an emerging class of porous, crystalline two- and three-dimensional (2D and 3D) polymers. Multivalent monomers polymerize through directional and reversible bonding to form ordered 2D and 3D frameworks with well-defined pores. Their pore sizes and functionality can be tuned through monomer design. Unlike traditional organic polymers, COFs exhibit long-range order and permanent porosity showing that they are promising for use in gas storage, separation, catalysis, sensors, energy storage, and optoelectronic devices. Our group is currently focusing on the following frontiers:
-
Developing a 2D Polymerization Toolkit Using Directional Bonding Approaches
-
COF Applications
-
Porous Cyclodextrin Polymers for Water Purification
-
Reshaping and Repairing Tough Polymers
-
Macrocycles

Developing a 2D Polymerization Toolkit Using Directional Bonding Approaches
The growth processes governing COF formation are not well understood, making it difficult to rationally synthesize higher quality materials. Our group has performed the first detailed mechanistic studies on COF growth. Detailed kinetic studies of both boronate ester-linked and imine-linked COFs revealed drastic differences between their growth mechanisms. Currently, we are using this mechanistic understanding to improve crystallinity and porosity in COF materials.
We have also synthesized discrete macrocycles to mimic the pore structure of 2D COFs for use as mechanistic analogues. These materials stack in a hexagonal lattice in the solid state but, unlike COFs, are dispersible in solution. This solubility allows us to directly investigate the stacking interactions between COF units with traditional analytical techniques such as NMR and GPC.
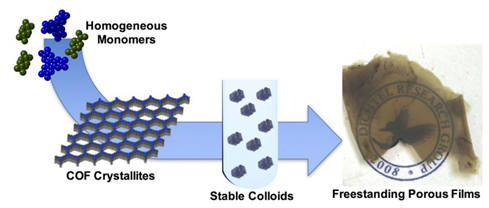
COF materials are typically formed as insoluble microcrystalline powders with poor processability. Ultimately, these materials need to be obtained as high quality thin films, large-area membranes, and dispersible colloids to take full advantage of their properties. As a first step towards this goal, we developed first syntheses of COFs as crystalline, oriented thin films on a monolayer graphene and have been continuously working on this problem ever since. Recently, we have developed flow technology to synthesize COF thin films with controllable thickness and low roughness such that they are ideal for device fabrication. Furthermore, we have stabilized monodispersed colloidal suspensions of COF crystallites using solvent additives. The size of these crystallites can be easily tuned and cast into free standing COF films, advancing the processability of these traditionally unprocessable materials.
COF Applications
Fundamental studies of COF syntheses benefit our research of COF applications in electrochemical supercapacitors, purification membranes, and optoelectronic devices. We have incorporated redox active sites into COFs to fabricate electrodes with rapid charging/discharging and excellent cyclability. We continued to polymerize conductive 1D polymers within the pores of a redox active COF. The composite materials exhibit high capacities and power densities for use as supercapacitors. To use COFs as purification membranes, we synthesize free-standing COF films and test their rejection of various water pollutants. We also study highly conjugated COFs in optoelectronic and photochemical devices. We are currently using spectroscopic and structural characterization techniques including femtosecond transient absorption (FTA) spectroscopy and grazing-incident wide angle X-ray scattering (GIWAXS) to understand the fundamental characteristics of photon-induced processes in COFs for use in devices such as photovoltaic cells.
Porous Cyclodextrin Polymers for Water Purification
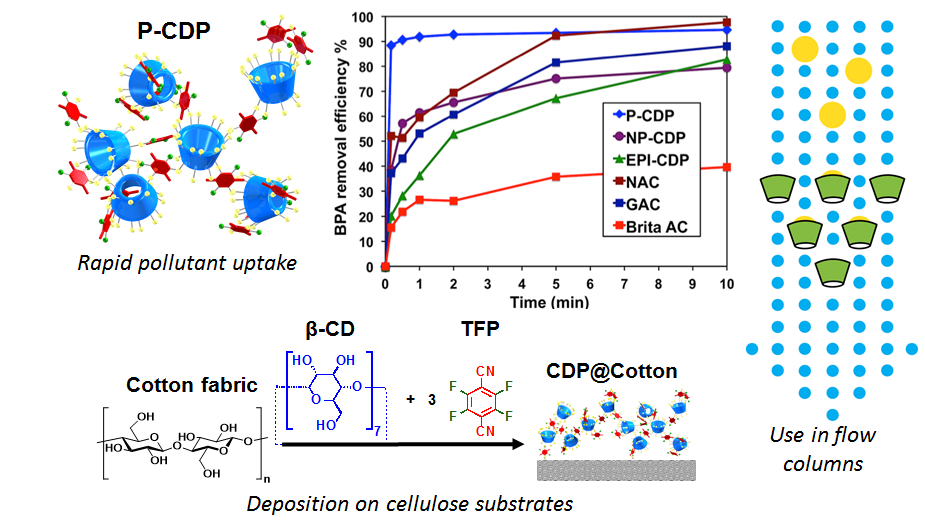
Organic micropollutants, including pharmaceuticals, pesticides, personal care products, and industrial chemicals, are emerging contaminants in global freshwater supplies. These compounds are not removed effectively by wastewater treatment plants, and their negative effects on the environment and human health are now coming into focus. We developed a porous, crosslinked polymer of β-cyclodextrin (P-CDP) to help combat this emerging global problem. P-CDP sequesters a large scope of these pollutants faster than the leading commercial materials and can be easily reused.
We currently focus on improving the performance of these materials as well as expanding their scope of applications. We perform model reaction studies to better understand structure-property relationship in P-CDP, enabling us to optimize the synthesis and performance of P-CDP and related polymers for micropollutant capture.
To further expand the use of P-CDP, we have developed a method for grafting the polymer onto a cotton fabric substrate. This new material (CDP@cotton) sequesters micropollutants not only from solution but also from gas, outperforming three commercial materials used for odor removal. This concept is now being applied to other substrates beyond cotton and tested in applications such as respirators, medical consumables, as well as analytical and sensing devices.
Furthermore, we are developing other cyclodextrin based polymers with enhanced affinity for certain micropollutants by expanding the crosslinker scope, optimizing synthesis conditions and post-processing methods.
Reshaping and Repairing Tough Polymers
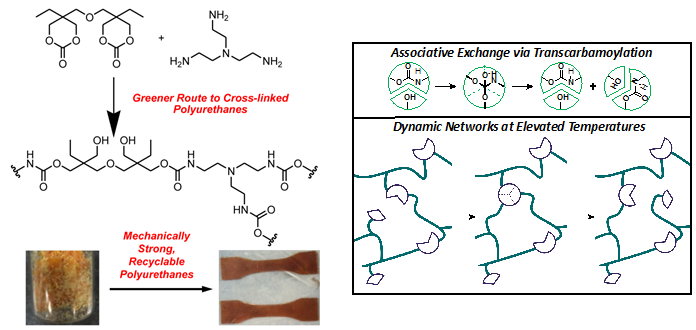
Thermosets are a class of durable, yet lightweight cross-linked polymers. Traditional thermosets, however, are neither repairable nor recyclable and must be disposed of after failure. Developing reprocessable and reshapable materials with comparable mechanical properties represents an ongoing challenge in polymer science. We are studying a class of dynamic polymers called vitrimers. Vitrimers are tough, crosslinked polymers that can change their shape by exchanging their crosslinks at elevated temperatures. This process provides polymers with the excellent mechanical properties of thermosets at normal service temperatures and the versatile reprocessing of thermoplastics at elevated temperatures.
Our initial investigations of these materials have focused primarily on polyhydroxyurethane vitrimers. In addition to their excellent mechanical properties, these materials can be reprocessed via compression molding at elevated temperatures. We have used extensive physical organic chemistry and polymer characterization to understand the processes responsible for repair in these materials. Current efforts include: the optimization of mechanical properties and reprocessing in these materials; the development of more sustainable and renewable feedstocks for vitrimers; the expansion of dynamic chemistries compatible with vitrimer design concepts; and applications of these materials that take advantage of their unique ability to flow while maintaining excellent mechanical properties. This work is supported by the National Science Foundation-funded Center for Sustainable Polymers.
Macrocycles
Nanotubes assembled from macrocyclic precursors offer unique properties that stem from their low dimensionality, structural rigidity, and tunable microenvironments through rational synthetic design. Despite these tailorable properties, realizing these structures are met with challenges in (1) the efficient synthesis of macrocycles capable of assembly, and (2) designing robust, directional interactions capable of stabilizing high-aspect ratio structures (>103), similar to what is seen in carbon nanotubes and various biological filaments. In the Dichtel group we use the tools of organic and supramolecular chemistry to overcome both of these challenges.
Traditional macrocyclization strategies rely on the stepwise construction of linear precursors, which are subsequently cyclized under dilute, low yielding conditions. In the Dichtel group, we use a dynamic bonding approach to macrocycle synthesis which is driven by supramolecular assembly of the macrocycles into periodic structures. This assembly event restricts imine exchange and drives the macrocyclization reactions to high yield.
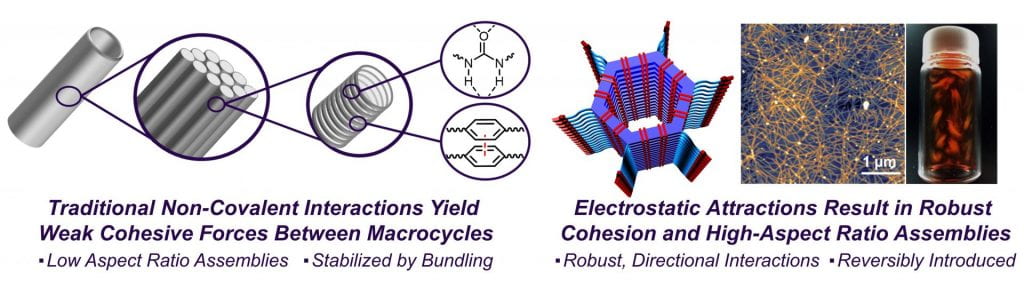
In terms of macrocycle assembly, supramolecular chemists have long targeted synthetic nanotubes by designing macrocycles capable of stacking, with inspiration undoubtably coming from covalently linked systems, such as carbon nanotubes, and various biological filaments, the most notable being actin. However, while these hallmark materials possess extremely high aspect ratios (>103), primitive systems based on macrocycle assembly were never able to realize that goal, as their noncovalent interactions were too weak to resist shear. Instead of forming isolated structures, columns of stacked macrocycles bundled into structures that were ten to hundreds of nanometers wide.
In the Dichtel group, we take advantage of our high-yielding imine-linked macrocycle synthesis to prepare a variety of functional macrocycles which can be assembled into high-aspect ratio nanotubes by protonation of the imines or other basic moieties. This protonation event turns on robust electrostatic and solvophobic interactions which drive assembly. Molecular dynamics simulations predicted the strength of these interactions to be on the order of carbon-carbon bonds. These simulations were experimentally validated by touch-spinning fibers of the nanotubes which possessed a higher Young’s modulus than traditional linear polymers.
Currently we are interested in using our knowledge of macrocycle assembly to develop a modular approach to highly functionalized structures. We believe that our expertise in organic, supramolecular, and materials chemistry will allow us to use this platform to target designed applications.
Representative Publications
- Chavez, A. D.; Evans, A. M.; Flanders, N. C.; Bisbey, R. P.; Vitaku, E.; Chen, L. X.; Dichtel, W. R. Eur. J. 2018, 24 (16), 3989-3993.
- Sun, C.; Shen, M.; Chavez, A. D.; Evans, A. M.; Liu, X.; Harutyunyan, B.; Flanders, N. C.; Hersam, M. C.; Bedzyk, M. J.; Olvera de la Cruz, M.; Dichtel, W. R. Natl. Acad. Sci. U.S.A. 2018, 115, 8883-8888.
- Wang, S.; Chavez, A.D.; Thomas, S.; Li, H.; Flanders, N.C.; Sun, C.; Strauss, M.J.; Chen, L.X.; Markvoort, A.J.; Bredas, J.L; Dichtel, W.R. Mater. 2019, 31, 7104-7111.
- Strauss, M.J.; Asheghali, D.; Evans, A.M.; Li, R. L.; Chavez, A.D.; Becker, M.L.; Dichtel, W.R. Chem. Int. Ed. 2019, 58 (41), 14708-14714.
- Strauss, M.J.; Evans, A.M.; Castano, I.; Li, R.L.; Dichtel, W.R. Chem. Sci., 2020, 11, 1957- 1963.
Former Projects:
-
New Methods to Access Crowded or Contorted Aromatic Compounds
-
Modifying the Surface Chemistry of Graphene and Other 2D Materials
New Methods to Access Crowded or Contorted Aromatic Compounds
Substituted polycyclic aromatic hydrocarbons (PAHs) exhibit desirable electronic and optical properties for photovoltaics and field effect transistors. However, accessing increasingly elaborate polysubstituted aromatic systems larger than benzene is synthetically challenging. For example, while a disubstituted benzene has only 3 regioisomers, a disubstituted naphthalene has 14 regioisomers. As the number of substitutions increases, this number of regioisomers increases dramatically, reaching over 3000 regioisomers for hexa-substituted naphthalenes. We use a modular and regioselective cycloaddition of disubstituted acetylenes to rapidly access novel substituted polycyclic aromatic architectures. Regioselective decorated naphthalenes are formed through cycloaddition of a substituted benzaldehyde onto a substituted acetylene, wherein the acetylene may be substituted with a variety of aryl, silyl, or halo groups. The modularity of this reaction provides rapid access to previously difficult-to-synthesize aromatic structures, including regioselective access to a wide range of polyhalogenated naphthalenes. These molecules serve as versatile building blocks for more complicated structures such as stereocongested PAHs, contorted hexabenzocoronenes, and ortho-arylene oligomers. Moreover, the high conversions observed with this reaction make it suitable for post-synthetic modifications on acetylene containing polymers.
Modifying the Surface Chemistry of Graphene and Other 2D Materials
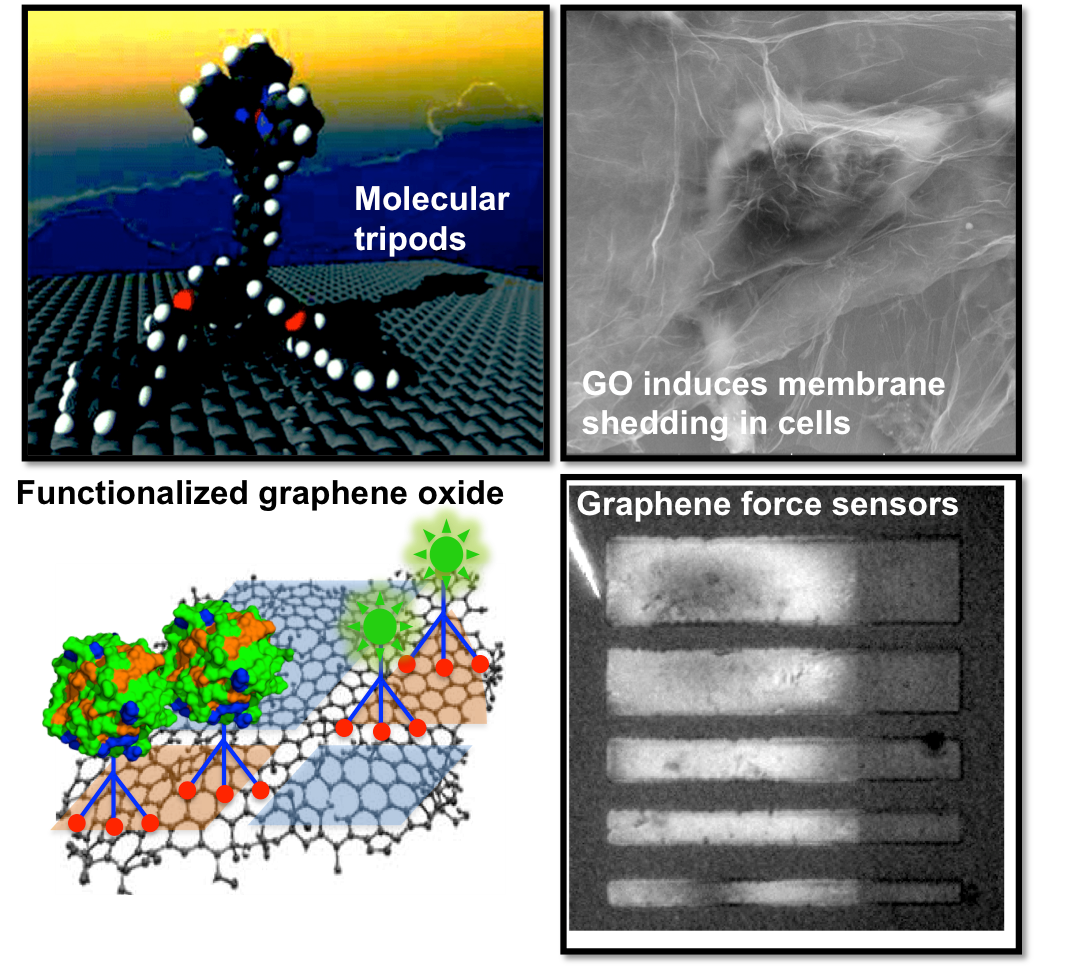
Our group develops graphene-based nano-medicine and nano-machines through surface functionalization and chemical control. We have synthesized molecular tripods to noncovalently functionalize pristine graphene. Molecular tripods exhibits excellent kinetic stability and intriguing diffusional surface dynamics on graphene(upper left). This strategy preserves the continuity of graphene’s hexagonal carbon network while imparting functionality for bioconjugation of enzymes, antibodies, and fluorophores. The tripodal strategy also allows us to explore the interface between biology and graphene oxide nanosheets (bottom left). Fluorescent labeling of graphene oxide (GO) reveals that GO nanosheets form complex and dynamic interfaces with mammalian cell plasma membranes, resulting in shedding of plasma membranes and changes of basic cell functions relevant to cancer therapeutics (upper right).
Smart use of molecular mechanisms on graphene also promises exciting opportunities in engineering graphene mechanics. Inspired by molecular tripods, we create sticky surfaces for graphene adhesion. We fabricate graphene force sensors and develop new force microscopy to study the supramolecular interactions governing graphene adhesion (bottom right). We also study reversible and responsive molecular mechanisms for control of graphene mechanical behaviour. Chemical control of graphene mechanics opens up opportunities for accessing the world’s thinnest origami robotics and nanomachines.