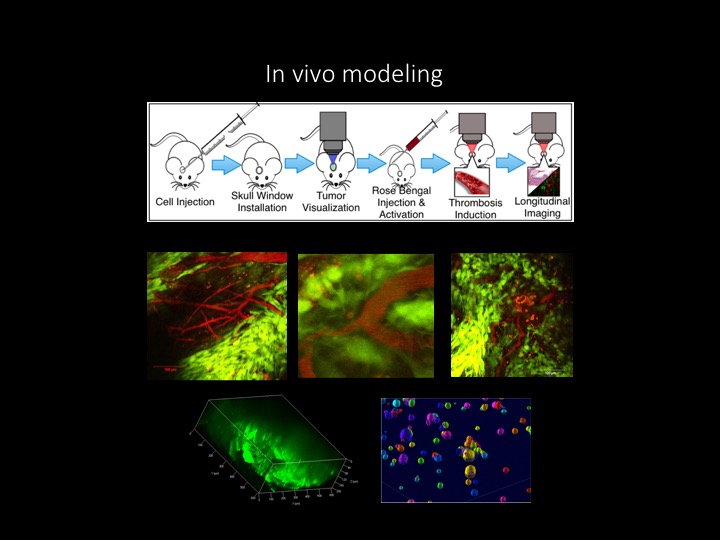
The Brat Lab investigates the biological underpinnings of glioma biology and is focused primarily on those mechanisms that lead to the most aggressive form of glioma, glioblastoma (GBM). We study genetics, hypoxia, stem cells and angiogenesis and use patient-derived samples as well as mouse and Drosophila models. Rapid growth of human gliomas is characterized by tumor necrosis, severe hypoxia and microvascular hyperplasia, a type of angiogenesis. We proposed that vaso-occlusion and intravascular thrombosis within a high grade glioma results in hypoxia, necrosis and hypoxia-induced microvascular hyperplasia in the tumor’s periphery, leading to neoplastic expansion outward. We are determining if transcriptional profiles, signaling networks and therapeutic targets vary within this altered micro-environment, and have also begun investigations involving the enrichment of glioma stem cells and influx of tumor associated macrophages. We use in vivo, multiphoton microscopy to monitor glioma dynamics following the onset of necrosis in a mouse model.
We also study mechanisms that confer specialized biologic properties to glioma stem cells (GSC) in GBM, including their ability to divide asymmetrically and their ability to home to hypoxic micro-environments. The Drosophila brain tumor (brat) gene normally regulates asymmetric cellular division and neural progenitor differentiation in the CNS of flies and, when mutated, leads to a massive brain containing only neuroblastic cells with tumor-like properties. We study the human homolog of Drosophila brat, Trim3, for its role in regulating asymmetric cell division and stem-like properties in GSCs. We are currently investigating those gene products that antagonize the tumor promoting effects of Brat/Trim3 loss on brain tumor development and are focused on CDK5/p35. For these studies, we use Drosphila as the primary model for discovery and translate findings to mouse xenograft models using patient-derived, genetically characterized gliomas.