Modeling Structural plasticity in the Olfactory System
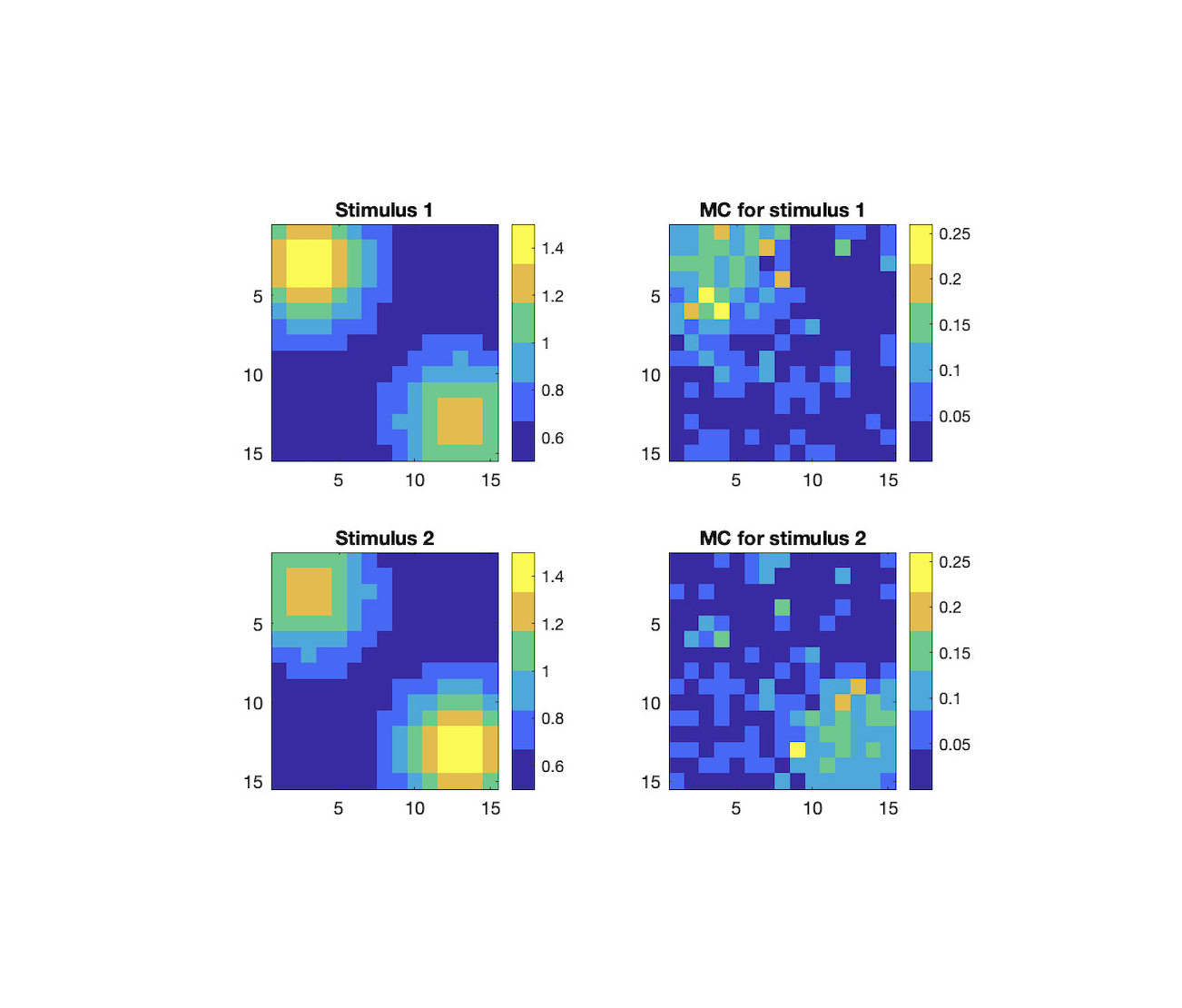
The olfactory bulb (OB) is one of the few areas in the mammalian brain which undergoes substantial structural changes in its neural circuitry well into adulthood. These changes, which include the formation and removal of synapses as well as the neurogenesis and apoptosis of interneurons, are presumed to play a key role in the perceptual learning and forgetting of animals upon repeated odor exposure. In the OB, excitatory projection neurons called mitral cells (MCs) form an odor representation which is shaped in part by a large population of inhibitory granule cells (GCs). Learning occurs when repeated presentation of a stimulus alters this odor representation through network changes which are driven by two forms of structural plasticity: the formation and removal of reciprocal MC-GC synapses and the birth and death of adult-born GCs (abGCs). The loss of memories on the other hand can be attributed to a combination of synaptic rewiring and the death of abGCs which helped code for certain odors. In this project, we present a biophysically-motivated computational model of structural synaptic plasticity and neurogenesis in the rodent OB to investigate synaptic integration of abGCs and their impact on learning and discrimination. In doing so, we illuminate factors which aid the integration of adult-born neurons into the OB and how the death of abGCs can facilitate learning.
