Faced with a growing global population, an aging domestic population, and limited natural resources, advanced materials represent a promising solution to challenges in clean energy and human health. The rapid identification and testing of materials with designed performance and properties is essential to technological advancement in a range of fields. Innovations at the interface of synthetic chemistry and materials science are necessary for materials discovery to keep pace with the evolving demands of the modern world.
The Kalow group is interested in discovering synthetic transformations that access organic materials, and controlling functional materials by manipulating reactivity. Macromolecules offer unique opportunities to translate molecular design into physical properties and structural features at the nano- to macroscale. By introducing energy in the form of light, we can spatiotemporally control monomer activation and tune materials’ properties and functions. A guiding principle in our research is the detailed understanding of reaction mechanism to drive optimization, discover new opportunities, and uncover general insights.
Applications of this research include optoelectronic and magnetooptic devices, sustainable polymers, and responsive hydrogels for biomedicine. Students will gain experience in method development, polymer synthesis, mechanistic analysis, and materials characterization and testing.
Learn more:
- POLY webinar with Brett Fors (Cornell) and Julia
- “Member Spotlight” about Julia by AAAS
Research areas
Reversibly photocontrolled polymer networks
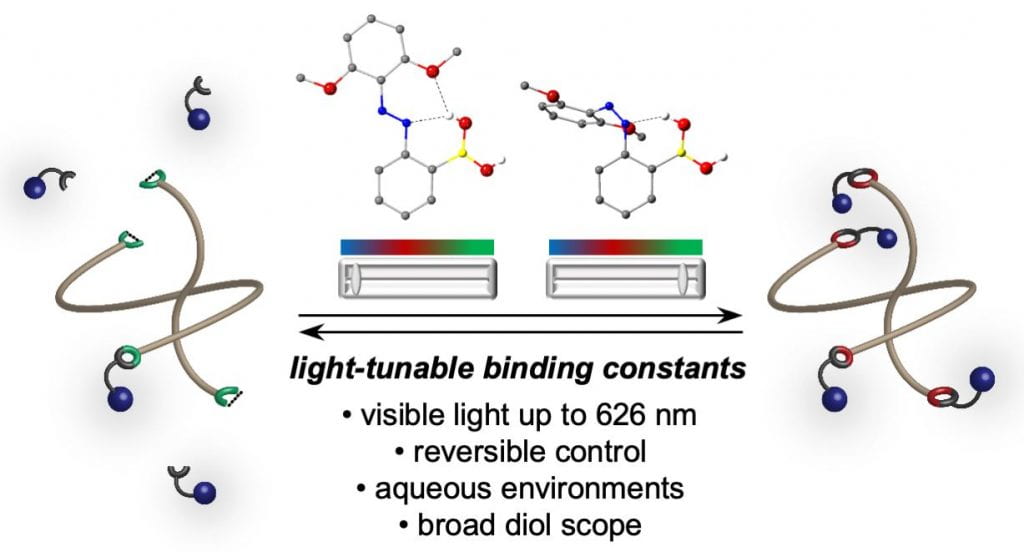
Polymer networks held together with static crosslinks are ubiquitous in modern life as gels, elastomers, and thermosets. If these crosslinks are dynamic instead of static, the polymer networks can be recycled and repaired, adapt to applied forces and stimuli, and mimic viscoelastic tissues. The Kalow lab studies polymer networks that are held together with dynamic covalent bonds. Appealingly, these dynamic networks are literally a network of chemical reactions, with well-defined kinetics and thermodynamics, benefitting from the strength and directionality of covalent bonds. Thus, the mechanical properties of these networks can be tuned by altering the reactivity of their crosslinks.
We have elucidated design principles that allow us to control the thermodynamics and kinetics of dynamic covalent reactions with photoswitches. By incorporating these photoswitchable dynamic covalent bonds into polymer networks, we can reversibly control their mechanics using different wavelengths of light, without consuming reagents or generating byproducts. We are currently studying the application of our reversibly photocontrolled hydrogels as scaffolds for 4D cell culture.
- Accardo, J. V.; Kalow, J. A.* “Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks.” Chem. Sci., 2018, 9, 5987–5993. [DOI:10.1039/C8SC02093K]
- Accardo, J. V.; McClure, E. R.; Mosquera, M. A.; Kalow, J. A.* “Using visible light to tune boronic acid–ester equilibria.” J. Am. Chem. Soc., 2020, 142, 19969–19979. [DOI:10.1021/jacs.0c08551]
- Barsoum, D. N.; Kirinda, V. C.;† Kang, B.;† Kalow, J. A.* “Remote-controlled exchange rates by photoswitchable internal catalysis of dynamic covalent bonds.” J. Am. Chem. Soc., 2022, 144, 10168–10173. [DOI:10.1021/jacs.2c04658]
- Kang, B.; Kalow, J. A.* “Internal and external catalysis in boronic ester networks.” ACS Macro Lett., 2022, 11, 394–401. [DOI:10.1021/acsmacrolett.2c00056]
- Perspective: Zhang, V.;† Kang ,B.;† Accardo, J. V.;† Kalow, J. A..* “Structure–reactivity–property relationships in covalent adaptable networks.” J. Am. Chem. Soc., 2022, 144, 22358–22377. [DOI:10.1021/jacs.2c08104] (†equal contribution)
Catalyst-free reprocessable elastomers
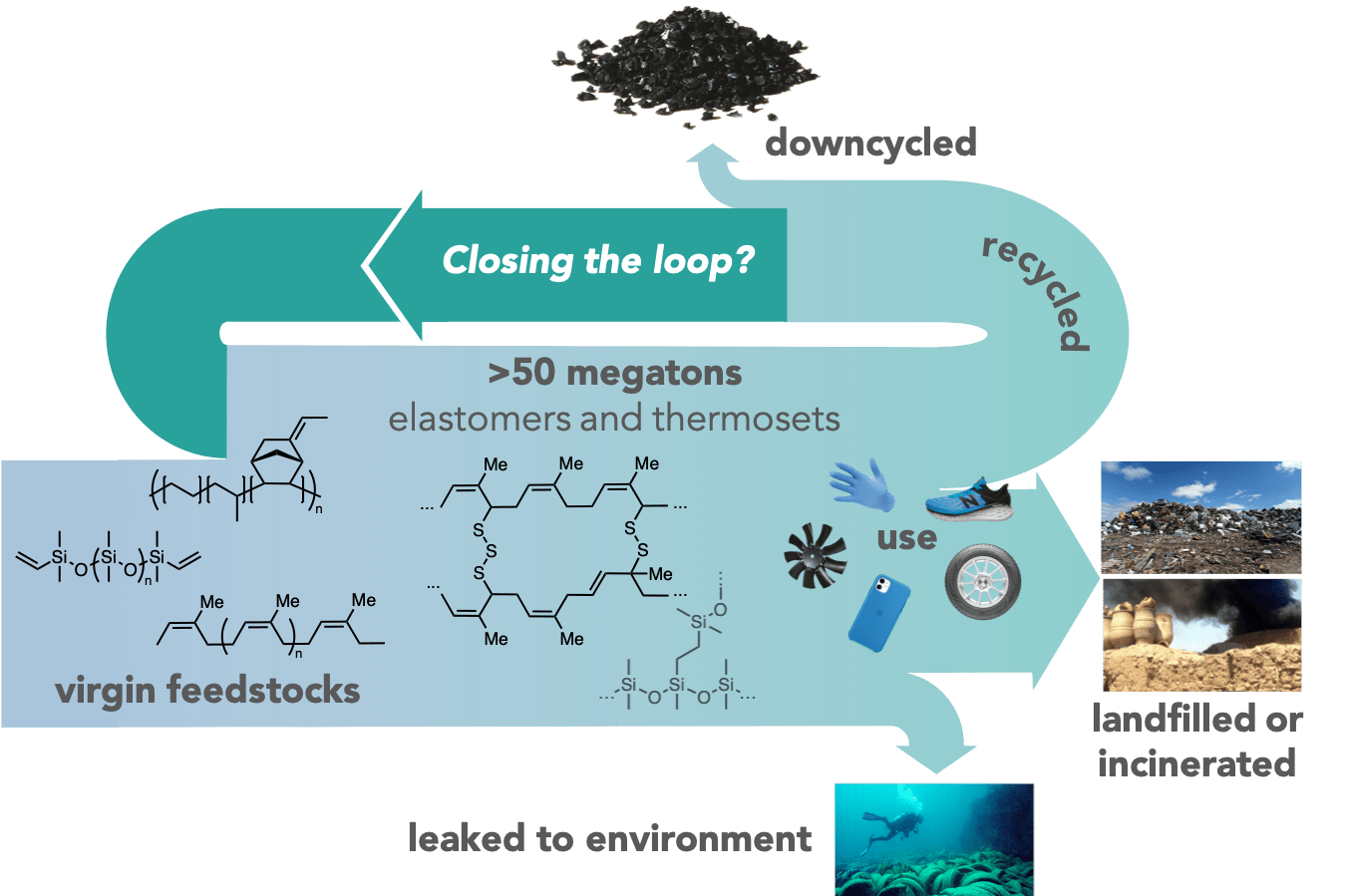
The permanent crosslinks of thermosets and elastomers provide excellent mechanical strength and solvent resistance, but hinder mending and recycling. Strategies to enable reconfiguration of the crosslinks under non-service conditions have significant promise for a circular plastics economy. Vitrimers are a class of dynamic covalent networks that feature exchange by associative (SN2-like) mechanisms. We have identified associative reactions for bond exchange under mild, catalyst-free conditions, and shown how the structure of the dynamic cross-linker and the polymer backbone can be manipulated to independently tune properties of interest. We are currently developing strategies to incorporate these dynamic cross-links into existing non-recyclable materials to impart reprocessability without compromising their properties.
- Ishibashi, J. S. A.; Kalow, J. A.* “Vitrimeric silicone elastomers enabled by dynamic Meldrum’s acid-derived crosslinks.” ACS Macro Lett., 2018, 7, 482–486. [DOI:10.1021/
acsmacrolett.8b00166] - El-Zaatari, B. M.; Ishibashi, J. S. A.; Kalow, J. A.* “Cross-linker control of vitrimer flow.” Polym. Chem., 2020, 11, 5339–5345. [DOI:10.1039/D0PY00233J]
- Ishibashi, J. S. A.; Pierce, I. C.; Chang, A. B.; Zografos, T.; Fang, Y.; Weigand, S. J.; Bates, F. S.; Kalow, J. A.* “Mechanical and structural consequences of associative dynamic cross-linking in acrylic diblock copolymers.” Macromolecules, 2021, 54, 3972–3986. [DOI:10.1021/acs.macromol.0c02744]
- Cho, N. H.;† El-Zaatari, B. M.;† Gokarn, R.; Wang, S.; Craig, S. L.; Kalow, J. A.*; Richards, J. J.* “Evaluating the Linear Viscoelasticity of Vitrimers Using Start-up and Cessation Tests.” [ChemRXiv DOI:10.26434/chemrxiv-2023-hb4v1]
Photochemical reaction discovery for organic materials

Conjugated polymers that transport electrons (n-type) are underdeveloped and challenging to prepare by living polymerizations. We have discovered that electron-deficient Grignard monomers undergo polymerization in the absence of a transition-metal catalyst, upon irradiation with visible light. The absence of transition metals avoids their deleterious effects in device applications. Experimental and computational studies have revealed a mechanism that relies on photoexcitation of the growing polymer chain. We envision that photochemical reaction mechanisms based on substrate photoexcitation will enable the selective functionalization of substrates with desirable photophysical properties within complex mixtures. These reactions will enable “Abiotic Directed Evolution”, an approach inspired by artificial selection, for the rapid discovery of new materials.
- Woods, E. F.; Berl, A. J.; Kalow, J. A.* “Photocontrolled synthesis of n-type conjugated polymers.” Angew. Chem. Int. Ed., 2020, 59, 6062–6067. [DOI:10.1002/anie.201915819]
- Review: Woods, E. F.†; Berl, A. J.†; Kalow, J. A.* “Advances in the synthesis of π-conjugated polymers by photopolymerization.” ChemPhotoChem, 2021, 5, 4–11. (†equal contribution) [DOI:10.1002/cptc.202000212]
- Woods, E. F.; Berl, A. J; Kantt, L. P.; Wasielewski, M. R.; Haines, B. E.;* Kalow, J. A.* “Light directs monomer coordination in catalyst-free Grignard photopolymerization.” J. Am. Chem. Soc., 2021, 143, 18755–18765. [DOI:10.1021/jacs.1c09595]
- Das, P.; Woods, E. F.; Ly, J. T.; Olding, J. N.; Presley, K. F.; Romanoff, B.; Grusenmeyer, T. A.; Weiss, E. A.; Kalow, J. A.* “Chemoselective nickel-catalyzed coupling through substrate photoexcitation.” [ChemRXiv DOI:10.33774/chemrxiv-2021-8wqv0-v2]
- Berl, A. J.; Sklar, J. H.; Yun, Y. J.; Kalow, J. A.* “Side-chain engineering in hydrophilic n-type π-conjugated polymers for enhanced reactivity.” ACS Macro Lett. 2023, 11, 503–509. [DOI: 10.1021/acsmacrolett.3c00085] [ChemRXiv DOI:10.26434/chemrxiv-2023-xss45]
Current support





Past support












Comments are closed, but trackbacks and pingbacks are open.