“There are some things you simply cannot prepare for,” I thought as the plane began the final descent over my home for the summer, the Netherlands. This first brush left me in awe– anxious to uncover the layers that make up this country’s unique character and rich past.
I had no idea that bike “traffic” existed, much less that there is a rush-hour for such traffic. I could not have anticipated being inches away from real-life actual Rembrandt paintings on our very first day. It is overwhelming to be in the presence of such revered masterpieces, and to contemplate how the artists of the Dutch Golden Age could achieve such strikingly realistic works. Even after seeing Girl with a Pearl Earring in person, I’m not sure I’ll ever understand how Vermeer was able to capture light in a bottle and apply it to canvas. Being in the center of this art hotspot feels like the luckiest day ever: as if you’d stumbled upon a whole field of four-leaf clovers, bathed overhead by a quadruple rainbow. Only in the Netherlands could I experience so much of the Dutch Golden Age. There have been so many once-in-a-lifetime experiences as I’ve learned about art conservation and Dutch cultural heritage.
The power of science to preserve these pieces is exhilarating. Admittedly, my background is in materials science and engineering, and so it has been eye-opening to be introduced to the art conservation field and its scientific, historical, cultural, and ethical components. I’ve learned it is a far cry from an easy task to preserve art; the endeavor seems quite chaotic when you stop to think of all the variables. What is the paint composition? What is the chemistry of the binders? How thick is each paint layer, and was it allowed to dry before the next layer was painted? What would the painting have looked like at the time of creation? This is just a tiny sample of questions that art conservators, art historians, and scientists must answer together.
As a student in the NU-IRES program, I conduct research at the University of Amsterdam (UvA) and make my own contribution to the art conservation community. My undergraduate research at Rutgers University heavily focused on characterization of polymer composites, and I became particularly interested in rheology. I am fortunate to continue studying rheology at Northwestern University as a graduate student in the Shull Group. Our group works extensively with the quartz crystal microbalance (QCM) to study polymer thin films. QCM is a powerful technique and—depending on film thickness– can be sensitive to mass changes and viscoelastic properties. At Northwestern, I have been using the QCM to investigate the effect of molecular weight on glass transition temperature in polystyrene thin films. A great advantage of the QCM is its portability. After my last final of the academic year, I packed the QCM equipment in a small cereal-sized box and took the lab on the road.
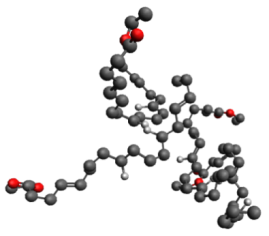
After landing in one of the art capitals of the world, it was time to shift to a material that art conservators and scientists really care about: linseed oil. I am investigating the QCM’s potential to study the early curing stages of this drying oil. My Dutch advisor, Dr. Piet Iedema, and his group have developed computational models to describe the mechanisms occurring in these early stages. I’m interested in developing a QCM experiment to study the changes in mass when a raw linseed oil film is applied to the QCM crystal. Linseed oil is composed of a hodgepodge of fatty acids; namely -linolenic, oleic, and linoleic acid. Atmospheric oxygen molecules react with the unsaturated bonds in these fatty acids and create highly reactive peroxide species, which then initiate a whole cascade of reactions. During these processes, the oxygen absorption corresponds to an increase in mass. The linseed oil also hardens during the cure process. I’m interested in using the QCM to monitor changes in both mass and viscoelasticity. Linseed oil has been more difficult than polystyrene to work with, and my equipment started malfunctioning. Fortunately, my best friend had planned to come visit in mid-July and was able to come with some replacement equipment from the Shull lab. But even with that stroke of luck, there’s always a risk of persistent problems. These various challenges provide great learning opportunities. So while I may not have been able to conduct as many experiments with linseed oil as I had hoped– I am still learning valuable information about QCM troubleshooting, circuits, mechanics, paintings, metal soaps, etc.
I am immensely grateful for the support of Dr. Shull, Dr. Iedema, Dr. Walton, and Dr. Gambardella this summer. Dr. Shull’s advising and troubleshooting help via email and weekly Skype calls has been a real lifeline. I thank him for his encouragement to apply for the NU-IRES program. The time here has been transformative in ways I could not have anticipated, but sometimes it’s fantastic to have your breath taken away.




 My name is Gabriela Diaz and I am a 3rd year student at Texas A&M University, Kingsville pursuing a Bachelor of Science in Chemical Engineering. My background in cultural heritage is a research experience (NSF-REU) at the University of North Texas studying X-ray fluorescence (XRF) to characterization 19th and 20th century silver-plated objects from the Dallas Museum of Art. I am here in Amsterdam through the Cultural Heritage Research in the Netherlands, an International Research Experience for Students (IRES) which is sponsored by the National Science Foundation (NSF).
My name is Gabriela Diaz and I am a 3rd year student at Texas A&M University, Kingsville pursuing a Bachelor of Science in Chemical Engineering. My background in cultural heritage is a research experience (NSF-REU) at the University of North Texas studying X-ray fluorescence (XRF) to characterization 19th and 20th century silver-plated objects from the Dallas Museum of Art. I am here in Amsterdam through the Cultural Heritage Research in the Netherlands, an International Research Experience for Students (IRES) which is sponsored by the National Science Foundation (NSF).
 So far I have learned that no matter how well you think you understand something there will always be something unexpected to bring you back to the scientific chase. I never imagined finding myself studying methods to analyze art inside a hospital, and yet as the days pass it makes perfect sense that skills applied in preserving the health of an individual can find some application in preserving the health of a great painting. Everyday brings new questions and new things to image under the scan-head of the OCT. This constant curious thrill as well as the immersion in the energy of the city has made my transition from the states an adventure to remember.
So far I have learned that no matter how well you think you understand something there will always be something unexpected to bring you back to the scientific chase. I never imagined finding myself studying methods to analyze art inside a hospital, and yet as the days pass it makes perfect sense that skills applied in preserving the health of an individual can find some application in preserving the health of a great painting. Everyday brings new questions and new things to image under the scan-head of the OCT. This constant curious thrill as well as the immersion in the energy of the city has made my transition from the states an adventure to remember.


 My name is Katherine Ferreras and I am a senior at The City College of New York (CCNY) of The City University of New York, pursuing a Bachelor of Science in Environmental Chemistry, and a minor in Earth and Atmospheric Science. This summer I am in Amsterdam as part of Cultural Heritage Research in the Netherlands, a NSF-funded International Research Experience for US Students (IRES) in the Netherlands. Under the mentorship of Dr. Urs Jans and close collaboration of Dr. Glen Kowach at my home institution, I have been able to explore and expand my interest in groundwater remediation techniques. My work focuses on the development of green rust, a highly reactive iron (II) mineral capable of reducing a range of inorganic and organic species. As this includes toxic materials and organic pollutants, this project can help to provide the necessary knowledge for a new approach to remediate contaminated aquifers. The motivation of my project is to understand the structure, behavior and reductive kinetics of the reaction between green rust and chlorinated hydrocarbons, since this is a possible effective approach to reduce nonpolar chemicals, such as carbon tetrachloride that are found in ponds at the bottom of aquifers.
My name is Katherine Ferreras and I am a senior at The City College of New York (CCNY) of The City University of New York, pursuing a Bachelor of Science in Environmental Chemistry, and a minor in Earth and Atmospheric Science. This summer I am in Amsterdam as part of Cultural Heritage Research in the Netherlands, a NSF-funded International Research Experience for US Students (IRES) in the Netherlands. Under the mentorship of Dr. Urs Jans and close collaboration of Dr. Glen Kowach at my home institution, I have been able to explore and expand my interest in groundwater remediation techniques. My work focuses on the development of green rust, a highly reactive iron (II) mineral capable of reducing a range of inorganic and organic species. As this includes toxic materials and organic pollutants, this project can help to provide the necessary knowledge for a new approach to remediate contaminated aquifers. The motivation of my project is to understand the structure, behavior and reductive kinetics of the reaction between green rust and chlorinated hydrocarbons, since this is a possible effective approach to reduce nonpolar chemicals, such as carbon tetrachloride that are found in ponds at the bottom of aquifers.





 name is Lindsay Oakley and I am a Northwestern University graduate student working in the Netherlands this summer as part of a NSF sponsored International Research Experience for Students (IRES) focused on investigating questions in cultural heritage science. I was first introduced to the field as an undergraduate at the College of William and Mary. When I was struggling to settle into a major, deciding between chemistry and history classes that I also enjoyed, I met a chemistry professor setting up a collaboration with the paintings conservator for Colonial Williamsburg. I volunteered to get involved and was quickly fascinated by the way that the scientific study of objects can reveal insights into people and technologies of the past. But historical knowledge also serves to help interpret scientific results, and this synergy leads to new understanding. This experience led me to Northwestern and a project in partnership with the Art Institute of Chicago where I have continued studying paint and new tools that can be applied to understand it and preserve it for future generations.
name is Lindsay Oakley and I am a Northwestern University graduate student working in the Netherlands this summer as part of a NSF sponsored International Research Experience for Students (IRES) focused on investigating questions in cultural heritage science. I was first introduced to the field as an undergraduate at the College of William and Mary. When I was struggling to settle into a major, deciding between chemistry and history classes that I also enjoyed, I met a chemistry professor setting up a collaboration with the paintings conservator for Colonial Williamsburg. I volunteered to get involved and was quickly fascinated by the way that the scientific study of objects can reveal insights into people and technologies of the past. But historical knowledge also serves to help interpret scientific results, and this synergy leads to new understanding. This experience led me to Northwestern and a project in partnership with the Art Institute of Chicago where I have continued studying paint and new tools that can be applied to understand it and preserve it for future generations.