Independent Research (PI: Tracy Lohr, ISEN Grant)
Bio-Alcohol Reforming
This project involves heterogeneous alcohol reforming of bio-available alcohols to hydrogen fuel and commercially valuable aldehydes. The grant involves laboratory space and research funds to build and operate a trickle-bed flow reactor. The goal of this project is to optimize conditions on the small scale for potential scale-up and commercialization. This work has been featured by ISEN and Northwestern Research (Link)
Projects that Tracy manages, directs, and supervises in the Tobin Marks Group (Subgroup A Team: Organometallics and Catalysis. Approx ~ 15 people including undergraduates, graduate students, and post-doctoral fellows. Tobin Marks A Team Subgroup):
Organo-f-element Hydroelementation
Lanthanides and actinides offer a new frontier in organometallic chemistry. Our interests include tuning electrophilic f-element centers to effect unusual types of transformations. Closely coordinated are computational and thermochemical investigations of bonding and bond energies. These offer deeper insight into bonding and aid in designing new reactions. With recent advances in lanthanide-mediated catalysis and bond-energy studies, we are developing f-element catalysts for hydrosilation, hydroalkoxylation, hydroamination, and hydrothiolation. Recently, we have focused on the hydroelementation of carbon-heteroelement bonds such as carbonyls and pyridines.
Catalysis for Biomass Conversion (C-O cleavage)
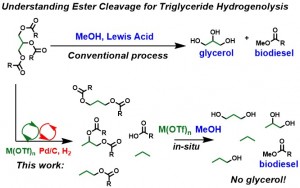
To utilize biomass as an energy source, not only with current infrastructure, but for maximum energy return, the oxygen content must be reduced. One method to achieve this is to develop selective catalytic methods to cleave C–O bonds commonly found in biomass (aliphatic and aromatic ethers and esters) for the eventual removal of oxygen in the form of volatile H2O or carboxylic acids. This Laboratory previously reported that recyclable “green” lanthanide triflates are excellent catalysts for C–O bond-forming hydroalkoxylation reactions. Based on the virtues of microscopic reversibility, the same lanthanide triflate catalyst should catalyze the reverse C–O cleavage process, retrohydroalkoxylation, to yield an alcohol and an alkene. However, ether C–O bond-forming (retrohydroalkoxylation) to form an alcohol and alkene is endothermic. Guided by quantum chemical analysis, our strategy is to couple endothermic, in tandem, ether (and analogous) ester C–O bond cleavage with exothermic alkene hydrogenation, thereby leveraging the combined catalytic cycles thermodynamically to form an overall energetically favorable C–O cleavage reaction. This work has been Highlighted in Chemistry World Magazine (Link)
Bimetallic Olefin Polymerization Catalysis
We are synthesizing group 4 and group 10 bimetallic polymerization catalysts. Ligand systems have been designed to keep two metal centers in close proximity. The resulting catalysts have high functional group tolerance and activity. Compared with monometallic analogues, the bimetallic systems have 2x the ethylene polymerization activity, 2x the branch density, and 3x greater selectivity for comonomer enchainment.
Supported Organometallics/Heterogeneous Catalysis
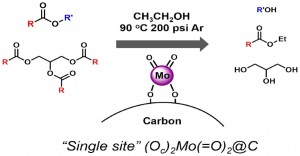
Our group is interested in the surface chemistry and reactivity of supported catalysts. Supporting molecular precursors onto supports can provide well-defined heterogeneous catalytic species. We are currently exploring single-site supported organo-zirconium catalysts that are highly active for ethylene polymerization and selective benzene hydrogenation, and single site molybdenum di-oxo catalysts for transesterification, alcohol oxidation, and carbonyl reductive coupling. In collaboration with Peter Stair’s group, we are also using supported organometallics and atomic layer deposition to produce well defined nanoparticles. We also have traditional heterogeneous projects focused on dry reforming and methane coupling. The molybdenum work has been highlighted by the Institute for Sustainability and Energy at Northwestern (ISEN) (Link).
Atomic Layer Deposition (ALD) Precursor Design
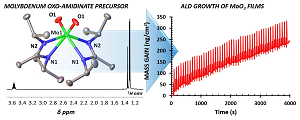
Atomic layer deposition is a method to produce conformal thin films. Through sequential A and B cycles, ALD is self-limiting, which allows for precise layer-by-layer growth. ALD requires volatile metallic precursors that are 1. Stable at high temperatures (up to 300 °C) and 2. Are highly reactive with the B cycle reagent (ex, H2O). Our group rationally designs new metallic, volatile ALD precursors for Mo, W, Mn, and Li for use in the preparation of Li/Mn batteries as well as in oxide and sulfide films for electronic applications. Work in this area is in collaboration with the Stair and Lauhon groups (NU), and Argonne National Laboratory.
Design of New Lubricant additives for Friction Reduction and Viscosity Modifications
In collaboration with the Wang and Chung groups (NU Mechanical Engineering), we are designing new lubrication additives for use in both fiction reduction and to modify viscosity. New silver additives prepared by our group show solid silver lubricant deposition at high temperatures, and amine based additives show a large reduction of friction in comparison to base oil in the boundary friction regime. The development of new viscosity modifiers is currently underway.